- Home/
- GATE MECHANICAL/
- GATE ME/
- Article
Zeroth Law of Thermodynamics
By BYJU'S Exam Prep
Updated on: September 25th, 2023

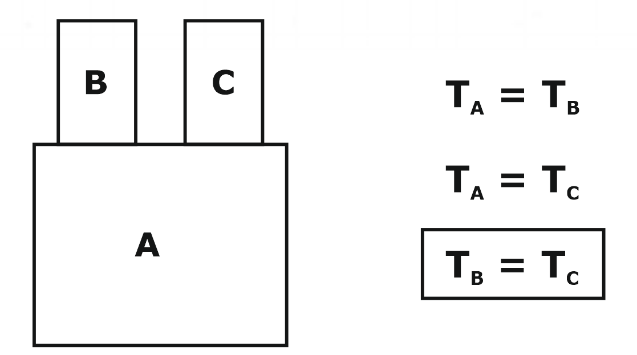
The Zeroth Law of Thermodynamics is a fundamental law of physics used to define temperature and establish a common temperature scale. It states that if two systems are in thermal equilibrium with a third system, they are in thermal equilibrium with each other. In other words, if system A is in thermal equilibrium with system B and system B is in thermal equilibrium with system C, then system A is in thermal equilibrium with system C.
The zeroth law of thermodynamics allows us to define temperature and measure it using a thermometer. A thermometer is a device that uses a thermometric property, such as the expansion of a solid or the pressure of a gas, to measure temperature. The zeroth law of thermodynamics is used to establish a common temperature scale, such as the Celsius scale or the Fahrenheit scale, which allows us to compare temperatures and express them in a standard unit of measurement. The zeroth law of thermodynamics is closely related to the first law of thermodynamics, a statement of energy conservation. The first law of thermodynamics is often used to understand the behavior of thermodynamic systems and to predict the changes in energy that will occur during various processes. Together, the zeroth and first laws of thermodynamics form the foundation of the science of thermodynamics, which studies the relationships between heat, work, and energy.
Something Interesting is coming on 15 Feb 2023. Register Yourself to Get Notified
Table of content
State Zeroth Law of Thermodynamics
The zeroth law of thermodynamics, a fundamental physics principle, establishes a recognized temperature scale. According to this rule, two systems are in thermal equilibrium with one another if they are also in thermal equilibrium with a third system. Zeroth Law of Thermodynamics is important for the GATE exam. To put it another way, if systems A and B are in thermal equilibrium with one another and systems B and C are in thermal equilibrium with one another, then systems A and C are as well.
The Zeroth Law of Thermodynamics is Often Written as
If system A is in thermal equilibrium with system B, and system B is in thermal equilibrium with system C, then system A is in thermal equilibrium with system C. This law allows us to define temperature and measure it using a thermometer. It is also used to establish a common temperature scale, such as the Celsius or Fahrenheit scale, which allows us to compare temperatures and express them in a standard unit of measurement. Overall, the zeroth law of thermodynamics is an important law of physics used to define temperature and establish a common temperature scale. It is closely related to the first law of thermodynamics, which is a statement of the conservation of energy, and together they form the foundation of the science of thermodynamics. It is the basis of temperature measurement.
Thermometric Property
A thermometric property is a physical property of a substance that changes predictably with temperature. Thermometric properties are used to measure temperature and are often used in thermometers to determine the temperature of a substance or system. Many different thermometric properties can be used to measure temperature. Some examples of thermometric properties include the expansion of solids and liquids, the resistance of electrical conductors, the pressure of a gas, and the intensity of electromagnetic radiation.
Thermometric properties are used in various thermometers, including mercury thermometers, alcohol thermometers, thermocouples, and infrared thermometers. Each type of thermometer uses a different thermometric property to measure temperature, and the accuracy and range of the thermometer depend on the specific thermometric property being used. Overall, thermometric properties are important for measuring temperature and are used in various thermometers and temperature-sensing devices.
Constant Volume Thermometer
From the ideal gas equation
PV∝ T
For constant volume: P α T, So T= f(P) Only
i.e., the temperature is only dependent on pressure; thus, for constant volume thermometer Pressure, P will be the thermometric property.
Constant Pressure Thermometer
From the ideal gas equation: PV∝ T
For constant Pressure: V α T, So T= f(V) Only
i.e., the temperature is only dependent on Volume; thus, for constant Pressure thermometer Volume, V will be the thermometric property.
(iii). The thermometric property for an electrical resistance thermometer is resistance.
(iv). In the case of a Thermocouple, Voltage or EMF will be thermometric property.
(v). In the thermometer case, length or Volume will be thermometric property.
These all are linearly varying.
Thus, T = a.N, where a is a random constant.
& N is the thermometric property
T1/T2 = N1/N2
T2 = N1/N2* T1
Five different kinds of the thermometer, each with its own thermometric property:
Energy
Energy is the ability to do work or cause change. It is a fundamental physical quantity that is present in many forms, such as kinetic energy (the energy of motion), potential energy (the energy of position or configuration), and thermal energy (the energy of heat).
Energy is important in many fields, including physics, engineering, biology and even for GATE ME question papers. It is a key element in studying thermodynamics, the science of the relationships between heat, work, and energy. Energy is also important in studying electricity, magnetism, and mechanics, and it plays a central role in the functioning of many technologies and devices. Energy can be transformed from one form to another but cannot be created or destroyed. This is known as the law of energy conservation, which is one of the fundamental laws of physics. Overall, energy is a fundamental physical quantity in many forms and is important in studying many fields and technologies.
First Law of Thermodynamics
The first law of thermodynamics is a statement of energy conservation, which states that energy cannot be created or destroyed, only converted from one form to another. It is often expressed in the form of a mathematical equation, which states that the change in the internal energy of a system is equal to the heat added to the system minus the work done by the system on its surroundings. This equation is known as the first law of thermodynamics and is written as ΔU = Q – W, where ΔU is the change in the system’s internal energy, Q is the heat added to the system, and W is the work done by the system on its surroundings. The first law of thermodynamics is a fundamental law of physics that applies to all physical systems, including thermodynamic systems, and is used to understand the behavior of systems and to design new technologies and devices that use energy.
Limitations of Zeroth Law of Thermodynamics
While the zeroth law of thermodynamics is a fundamental law of physics that is widely accepted and has many important applications, it does have some limitations. Some of the limitations of the zeroth law of thermodynamics include the following:
-
It does not define the concept of temperature: The zeroth law of thermodynamics does not define or provide a precise mathematical definition of temperature. Instead, it establishes a common temperature scale and allows us to compare temperatures and express them in a standard unit of measurement.
-
It only applies to systems in thermal equilibrium: The zeroth law of thermodynamics only applies to systems in thermal equilibrium, which means that they have reached a state of balance in which there is no net heat flow between the systems. It does not apply to systems not in thermal equilibrium, such as systems undergoing a change of state or undergoing a process in which heat is added or removed.
-
It does not provide a complete description of the behavior of physical systems: The zeroth law of thermodynamics only describes the relationships between temperature and thermal equilibrium. It does not provide a complete description of the behavior of physical systems and does not consider other factors, such as the motion and interactions of particles.
Get complete information about the GATE exam pattern, cut-off, and all those related things on the BYJU’S Exam Prep official youtube channel.



