- Home/
- GATE MECHANICAL/
- GATE ME/
- Article
Surface Tension
By BYJU'S Exam Prep
Updated on: September 25th, 2023

Surface Tension is the tendency of fluid surfaces to shrink to the smallest possible surface area. Surface tension is caused by the higher attraction of liquid molecules to each other (owing to cohesion) than air molecules at liquid–air contacts (due to adhesion). There are two basic factors at work in this situation. One causes the liquid to compress due to an inward push on the surface molecules. The second is a tangential force parallel to the surface of the liquid. The surface tension is the name given to this tangential force. As a result, the liquid appears to be encased in a stretched elastic membrane.
Have you ever noticed that once you’ve filled a glass full of water, you may still add a few more drops till it overflows? Have you ever broken a thermometer and watched how the mercury reacts when it falls? All of this is caused by the surface’s surface tension. Let’s look at the definition of surface tension, along with the SI unit, formula, and examples of surface tension.
Table of content
What is Surface Tension?
Surface tension is the phenomenon that occurs when the surface of a liquid comes into contact with another phase (it can be a liquid as well). Liquids prefer to have the smallest possible surface area. The surface of the liquid behaves like an elastic sheet. The surface tension of a liquid is primarily determined by the forces of attraction between the particles within the liquid and the gas, solid, or liquid in contact with it, according to the definition.
Surface Tension Definition
“Surface tension is the tension in a liquid’s surface film induced by the bulk of the liquid’s attraction to the particles in the surface layer, which seeks to minimize surface area.”
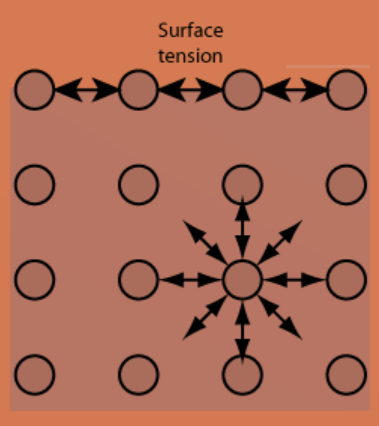
Figure: Surface Tension
Surface tension is determined by the forces of attraction between particles within a liquid and the forces of attraction of solids, liquids, and gases in contact with it. The work or energy necessary to remove the surface layer of molecules in a unit area is roughly equivalent to the energy responsible for surface tension phenomena. Surface tension is usually expressed in dynes/cm, the force necessary to break a 1-centimetre film.
The surface tension of several liquids is listed in the table below:
|
Liquid |
Surface Tension (N/M) |
|
Hydrogen |
2.4 |
|
Helium |
0.16 |
|
Water |
0.072 |
|
Sodium Chloride |
114 |
What is the Surface Tension Formula?
Intermolecular forces such as the Van der Waals force bind the liquid particles together. The particles are drawn toward the rest of the liquid along the surface. The ratio of the surface force F to the length L along which the force acts is known as surface tension.
Mathematically, The following is a formula for surface tension:
T=F/L
Where
- F stands for force per unit of length.
- L is the length of force action.
- T is the liquid’s surface tension.
What is the Unit of Surface Tension?
The SI unit for Surface Tension is the Newton per Meter (N/m). Other units can be found in the table below.
|
SI Unit |
N/m |
|
CGS Unit |
Dyn/cm |
Surface Tension Dimensional Formula
The formula of Surface Tension is
Surface tension = F/L.
We know that F = ma, thus we may replace the value in the equation
Surface Tension = ma/L.
We get = MLT-2L1 by putting the essential quantities into the equation.
We get =MT-2 by solving further.
As a result, the surface tension dimensional formula is MT-2.
Surface Tension Examples
Tiny insects called water striders might walk on water because their weight is insufficient to breach the water surface. There are numerous examples of surface tension in nature, like this one. The following are typical examples:
- Walking insects on the water
- A needle is floating on the water’s surface.
- Water will bridge the holes in rainproof tent fabrics because of the surface tension of the water.
- Jaundice diagnostic test
- Disinfectants reduce surface tension (disinfectants are solutions for low surface tension).
- Soaps and detergents are used to clean garments, lowering the water’s surface tension.
- When the surface tension of the water serves as the wall tension, water bubbles form in a round shape.
- This phenomenon also influences the form of liquid droplets.
How to Calculate Surface Tension?
Here’s an example of how to use the formula to calculate surface tension. We will find out how to calculate the surface tension on a liquid with a 7 N dragging force and a 2 m length.
Solution: F = 7 N, L = 2 m is the given value.
According to the surface tension formula:
T = F/L
Putting the given values;
T = 7/2
T = 3.5 N/m
Hence the surface tension on the given liquid is 3.5 N/m.
Methods of Measurement of Surface Tension
There are various methods by which we can calculate the surface tension acting on a fluid. Some examples of surface tension measurement methods are as follows:
- Spinning drop method
- Pendant drop method
- Du Noüy–Padday method
- Du Noüy ring method
- Wilhelmy plate method
- Pendant drop method
- Stalagmometric method
- Capillary rise method
- Bubble pressure method
- Resonant oscillations of a spherical and hemispherical liquid drop
- The vibrational frequency of levitated drops
- Sessile drop method


