- Home/
- GATE MECHANICAL/
- GATE ME/
- Article
Vapor and Gas Refrigeration System and Heat Pump Cycles
By BYJU'S Exam Prep
Updated on: September 25th, 2023

The essence of the refrigeration system and air-conditioning system is transferring heat from one location to another. A refrigeration system removes heat from a low-temperature area (such as a refrigerator or freezer) and transfers it to a higher-temperature area (such as the room or outdoor air). An air-conditioning system removes heat from indoor air and transfers it to outdoor air. Both systems use a refrigerant, a substance that can easily change from a liquid to a gas and back again, to absorb and release heat as it cycles through the system.
Refrigeration systems are used to cool down a space or to keep items at a specific temperature. They work by removing heat from one area and transferring it to another. While Air-conditioning systems are used to cool and dehumidify the air in a building or a vehicle. They work by removing heat from the air and transferring it to the outside. Let’s take a look into the concepts related to the refrigeration system and air-conditioning system.
Table of content
Refrigeration Cycle
The refrigeration cycle is a process used to transfer heat from a lower-temperature space or object to a higher-temperature space or object. It is commonly used in refrigerators, air conditioners, and other cooling systems. The refrigeration cycle begins when the refrigerant is in a low-pressure, low-temperature vapour form. It is then drawn into the compressor, which is compressed and heated.
The compressed, hot refrigerant then passes through the condenser, giving off its heat to the surrounding environment and condenses into a high-pressure, high-temperature liquid. The refrigerant flows through an expansion valve, allowing it to expand and cool. As it expands, it absorbs heat from the surrounding environment and evaporates into a low-pressure, low-temperature vapour. The refrigerant then returns to the beginning of the cycle, where it is drawn back into the compressor to repeat the process. Heat transfer from the lower-temperature space or object to the higher-temperature environment is what cools the space or object.
Heat Pump
A heat pump is a device that uses a small amount of energy to move heat from one location to another. It can heat and cool a space or object by transferring heat from the environment or inside a building to the desired location. Heat pumps use a refrigerant that absorbs heat from the environment or inside a building and then releases it into the desired location. The refrigerant is circulated through a closed system of pipes and coils and undergoes phase changes to absorb and release heat.
Heat pumps can operate in either direction, making them useful for heating and cooling applications. In the heating mode, a heat pump absorbs heat from the environment and releases it inside a building. In cooling mode, it absorbs heat inside a building and releases it to the environment. Heat pumps are typically more energy efficient than traditional heating and cooling systems because they do not generate heat or cold but rather move it from one location to another.
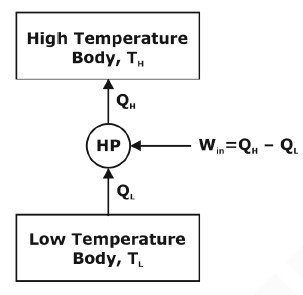
Coefficient of Performance:
![]()
Here, the desired effect is maintaining the body at a temperature higher than the surrounding.
Thus,
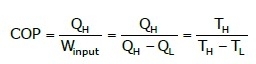
where:
TH = High-temperature body
TL = Low-temperature body
Refrigerator
A refrigerator is a household appliance that keeps food and other perishable items fresh by maintaining a temperature below the ambient temperature. It uses a refrigeration cycle to transfer heat from the inside of the refrigerator to the surrounding environment. The refrigeration cycle begins when the refrigerant is in a low-pressure, low-temperature vapour form. It is then drawn into the compressor, which is compressed and heated. The compressed, hot refrigerant then passes through the condenser, giving off its heat to the surrounding environment and condenses into a high-pressure, high-temperature liquid.
The refrigerant flows through an expansion valve, allowing it to expand and cool. As it expands, it absorbs heat from inside the refrigerator and evaporates into a low-pressure, low-temperature vapour. The refrigerant then returns to the beginning of the cycle, where it is drawn back into the compressor to repeat the process. The transfer of heat from the inside of the refrigerator to the surrounding environment is what keeps the inside of the refrigerator cool.



