DMSO Full-Form: Introduction, Production, Structure and Its Uses!!
By BYJU'S Exam Prep
Updated on: September 13th, 2023

DMSO Full-Form: Generally, students are keen to know about the different short forms that help them get the knowledge and help them secure good marks in the main exam. These short forms have been asked directly by the examiner in the exam. We have come up with articles that will help you to understand the different short forms. In this article, we will discuss the DMSO, its production, structure and uses. Read the full article and do not miss any of the parts to get detailed information about DMSO.
Table of content
Dimethyl Sulfoxide (DMSO)
DMSO Full Form: What is Dimethyl Sulfoxide (DMSO)?
It is an organosulfur compound with the formula (CH3)2SO. It is a colourless liquid, and it is a polar aprotic solvent which means it can dissolve both polar and nonpolar compounds. It is generally available as prescription medicine. It can be applied to the skin or can be taken by mouth and even can be injected into the veins. It comes from a substance that is found in wood. It is a by-product of papermaking.
DMSO Full Form: Production of DMSO
It is produced industrially by dimethyl sulphide which is a by-product of the Kraft process. Its production takes place in the atmosphere at the rate of 20-60 billion pounds per year. It is also present in soil and natural water. Its metabolism in soil (by microorganisms) helps in the formation of sulphur along with dimethyl sulphide.
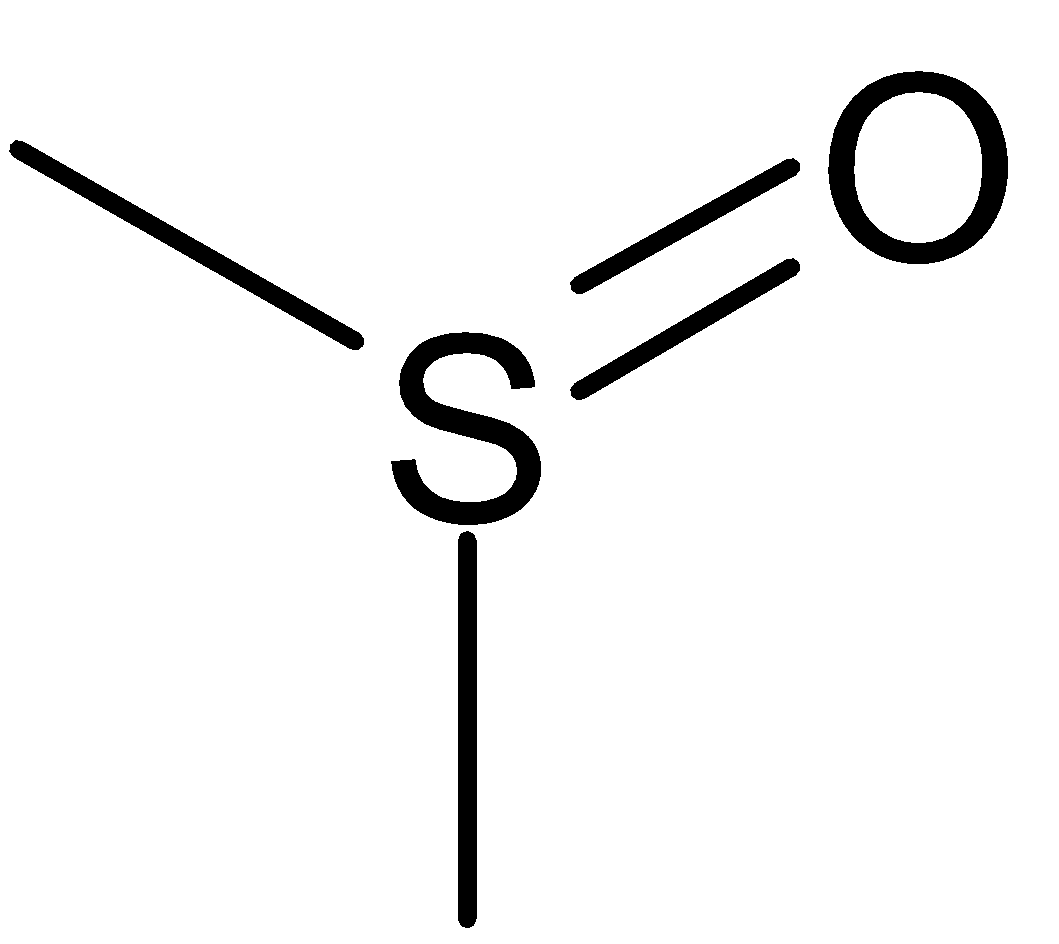
DMSO Full Form: Structure of DMSO
Its 2-D structure is:
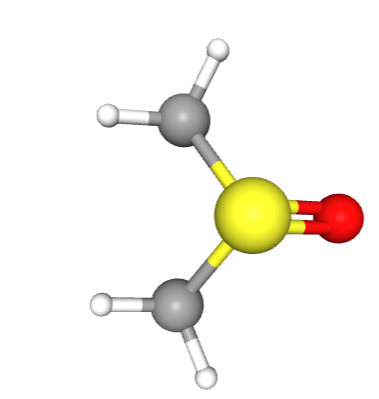
Its 3-D structure is:
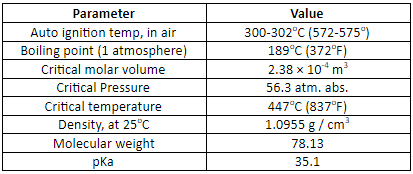
Properties:
DMSO Full Form: Reactions of DMSO
The sulphur centre present in DMSO acts as a nucleophile towards soft electrophiles and O acts as a nucleophile towards hard electrophiles. Its reaction with methyl iodide can be represented as:
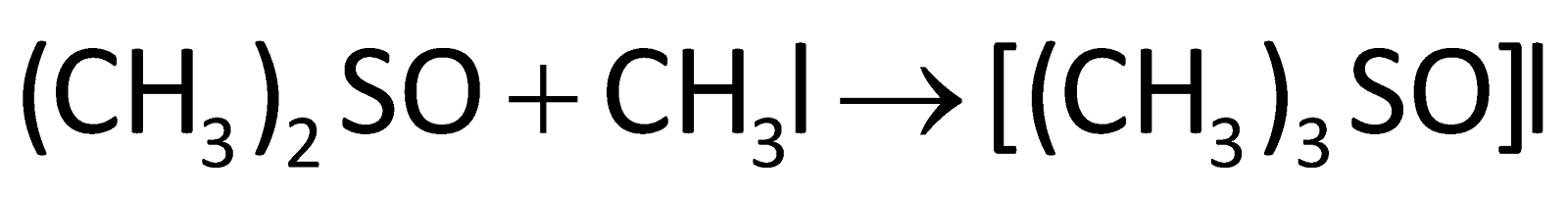
It is also used in Swern oxidation in which oxidation of primary or secondary alcohol to aldehyde takes place in the presence of DMSO. It acts as a base during the deprotonation of ketones, phosphonium salts and formamidinium salts.
Uses of DMSO:
It is used to relieve the pain of osteoarthritis. It can also be used as an alternative in the treatment of cancer. It is used to treat various conditions, and these are listed below:
- Eye problems
- Scars
- Headache
- Rheumatoid arthritis
DMSO Full-Form – Download the PDF Now
More Full Form Articles:
| DMSO Full Form | MOT Full Form |
| DMF Full Form | ECG Full Form |
I hope the above article was helpful for you. Let me know your feedback in the comments section below!!!
Check Out:
- Previous Year’s Papers for CSIR-NET Exam: Attempt Here
- Study Notes for CSIR-NET Chemical Science – Download PDF Here
- Study Notes for CSIR-NET Life Science – Download PDF Here
Stay Tuned for More Such Articles !!
Best of Luck!!
Sahi Prep hai to Life Set Hai!!
Go BYJU’S Exam Prep



