- Home/
- CDS & Defence/
- Article
The Number of Sigma and pi bonds in Benzene are
By BYJU'S Exam Prep
Updated on: September 25th, 2023
- 3, 3
- 3, 6
- 12, 3
- 12, 6
- None of the above / More than one of the above
The numbers of sigma and pi bonds in benzene are 12, 3. There are 6 C-H and 6 C-C bonds, just like in the structure. As a result, there are 12 sigma bonds. In benzene, there are three C=C pi bonds. Therefore, there are a total of 12 sigma bonds and 3 pi bonds. Thus, there are 15 covalent bonds in benzene.
Numbers of Sigma and Pi bonds in Benzene
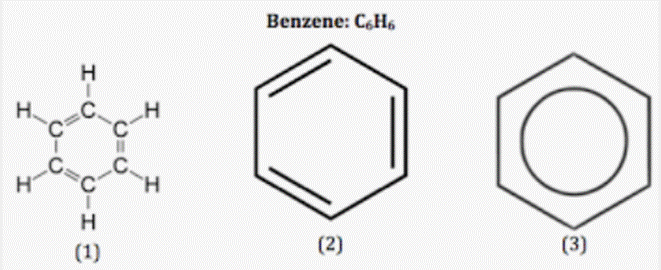
- The most basic aromatic hydrocarbon is benzene.
- Faraday discovered it in 1825.
- C6H6 is a molecular formula.
- 78.11 g/mol is the molar mass.
- 5.5 °C is the melting point.
- 80oC is the boiling point.
- It is extremely stable and highly combustible.
- It is present at room temperature in liquid condition.
- It has no color.
- It is an organic component of crude oil utilized in the dry cleaning industry as a solvent for fats, resins, and other materials.
- It is also known as “Benzol” and used as motor fuel.
- It has a high level of toxicity.
- Its excessive use raises the risk of cancer and contributes to bone marrow failure.
- The continuous cyclic pi link between the carbon atoms in benzene is what gives it its aromatic properties.
- Basically, it’s used to make polystyrene.
Table of content
Summary:
The Number of Sigma and pi bonds in Benzene are 1.3, 3, 2. 3, 6, 3. 12, 3 4. 12, 6 5. None of the above / More than one of the above
There are 12 sigma bonds and 12 pi bonds in benzene. 6 C-H and 6 C-C bonds are present, just like in the structure.
Related Questions:-
- What is the Dimensional Formula of Strain?
- Which Country will Host the 2023 Cricket World Cup?
- Which of the following can be Recycled? 1. Natural Oil 2. Coal 3. Natural gas 4. Metals
- Which of the following has the Highest Density? 1. Coke 2. Graphite 3. Charcoal 4. Diamond
- Which one of the following is NOT a Technique of Inventory Control?
- With Reference to the history of India, the terms Kulyavapa and Dronavapa denote
