What is a Double Displacement Reaction?
By BYJU'S Exam Prep
Updated on: September 13th, 2023
The double displacement reactive is a form of reaction where two compounds react to the exchange of ions in order to form two new compounds. In this reaction, the positive ions are the partner of negative ions. It is expected that the ionic compounds that get dissolved in water are the ones where the double displacement reaction takes place. Here is the reaction to represent the double displacement Reaction.
In a double displacement reaction, the atoms belonging to the two different compounds switch their places. The reactants are two compounds whereas, the products are two different compounds. Such as-
Fe2O3 + 6HCl → 2FeCl + 3H2O
The Double displacement reactions can be further classified as precipitation and neutralization reactions.
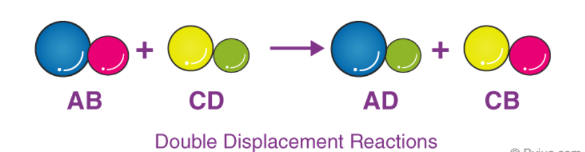
Table of content
Application of Double Displacement Reaction
Double Displacement Reaction is used in many industries to get the desired result. There are three different types of reactions-
- Acid indigestion
- Flame photometry
- Extraction of metals
Summary:
What is a Double Displacement Reaction?
Double Displacement Reactions are used when one chemical needs to be either oxidized or reduced by using another chemical. The reaction taking place between silver nitrate and sodium chloride is an ultimate example of a double displacement reaction. The silver trades its nitrite ion for the sodium chloride ion, this causes the sodium to pick up the nitrate anion.
AgNO3 + NaCl → AgCl + NaNO3
BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + 2 NaCl(aq)
☛ Related Questions:


