- Home/
- CDS & Defence/
- Article
Which of the following has the Maximum Number of Unpaired Electrons? 1. Mg2+ 2. Ti3+ 3. V3+ 4. Fe2+
By BYJU'S Exam Prep
Updated on: September 25th, 2023
- Mg2+
- Ti3+
- V3+
- Fe2+
Fe2+ has the maximum number of unpaired electrons because they have valence electrons in the d-orbital, the d-block elements are also known as transition metals.
Table of content
Why Fe2+ has the Maximum Number of Unpaired Electrons
- In the periodic table, d-block elements are situated between s-block and p-block elements.
- Because they behave in a way that transitions between s-block and p-block components, these d-block elements are known as transition elements.
- D-block element valence shell electronic configuration is (n- 1)d1-10 ns1-2.
- The 3d series, 4d series, and 5d series are three different sets of transitional elements.
- 3D series begins with Scandium (Sc) and end with Zinc (Zn).
- From Yttrium (Y) through Cadmium, the 4d series begins (Cd).
- Except for Cerium (Ce) and Lutetium, the 5d series begins with Lanthanum (La) and ends with Mercury (Hg) (Lu).
The table below lists the ions’ electrical configurations and the number of unpaired electrons in each one:
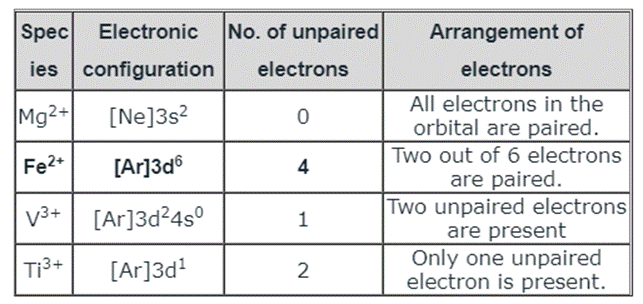
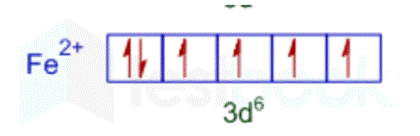
We can see from the preceding graph that Fe2+ contains the most unpaired electrons, four of which are distributed as follows:
Summary:-
Which of the following has the Maximum Number of Unpaired Electrons? Mg2+ Ti3+ V3+ Fe2+
The maximum number of unpaired electrons is present in Fe2+. It contains valence electrons in d-orbital and are called transition metals.
Related Questions:-
- If a + b = 4, and ab = 3, then what is the value of a3 + b3 = ?
- Moss and Ferns Reproduce by
- Sand Drains are Used to
- The Half-life Period of a Radioactive Substance Depends Upon
- Which is the Most Hazardous Metal Pollutant of Automobile Exhaust?
- Who Among the following is the Person in Charge of the Police Station?
