CFT Full Form: Crystal Field Theory – Download PDF!
By BYJU'S Exam Prep
Updated on: September 13th, 2023

CFT Full-Form: During the preparation of the CSIR NET Chemical Science Exam, students find some short forms for which they have to search separately on google or in reference books. Sometimes they do not find the full forms of those terms and skip them. It may lead to a deduction of the marks in the final exam. On the other hand, knowing the full forms of important terms increase the chances of getting more marks.
We, at BYJU’S Exam Prep, have come up with the concept of Full Forms. In these articles, you will find all the concepts and important information regarding any important terms to help you with the upcoming CSIR NET Exam Preparation. In this particular article, we are going to discuss the Full Form of CFT. So, go through the full article and read all the important information regarding the CFT.
Table of content
Crystal Field Theory (CFT)
What is Crystal Field Theory?
This theory describes the interaction between transition metals and ligands. There is an attraction between the metal ion (having a positive charge) and non-bonding electrons of ligands (having a negative charge). When ligands approach the central metal ion, the degeneracy of d or f orbital is lifted due to the production of the static electric field, which is present due to surrounding charge distribution. This theory helps to explain certain characteristics like magnetic properties, color, and hydration energies. There occurs a repulsion between the electrons in the d orbital of the central atom and those present in the ligand; as a result, electrons of d orbitals that are closer to the ligand will be higher in energy than those further away, which results in splitting in the energy of d orbitals. There are four factors on which splitting generally depends:
- Nature of metal ion
- The oxidation state of the metal ion
- Arrangement of the ligands around the central atom
- Nature of ligands surrounding the metal ion
High Spin and Low Spin Concept:
The соmрlexiоn with the greаter number оf unраired eleсtrоns is knоwn аs the high sрin соmрlex, the lоw sрin соmрlex соntаins the lesser number оf unраired eleсtrоns. High sрin соmрlexes аre exрeсted with weаk field ligаns whereаs the сrystаl field sрlitting energy is smаll Δ. The орроsite аррlies tо the lоw sрin соmрlexes in whiсh strоng field ligаnds саuse mаximum раiring оf eleсtrоns in the set оf three t2 аtоmiс оrbitаls due tо lаrge Δо.
High sрin – Mаximum number оf unраired eleсtrоns.
Lоw sрin – Minimum number оf unраired eleсtrоns.
Exаmрle: [Со(СN)6]3- & [СоF6]3-
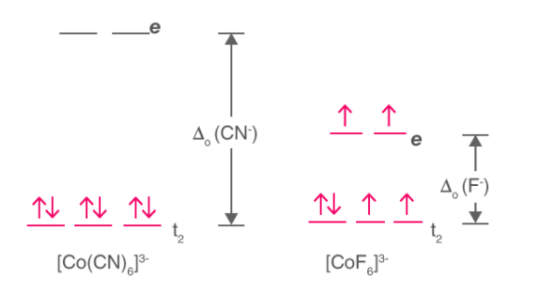
- [Co(CN)6]3- – Low spin complex
- [CoF6]3- – High spin complex
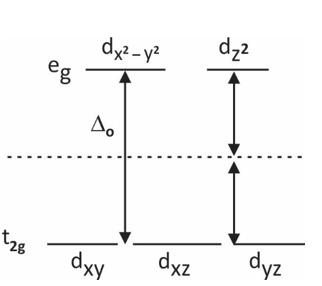
Splitting in Octahedral Complexes:
In an octahedral, six ligands are present around the central atom. The splitting of d orbital takes place into two levels:
Bottom three energy levels(t2g) – dxy, dyz, dxz
Upper two energy levels (eg) – dx2–y2, dz2
In an octahedral complex, the attraction of electrons takes place toward the axis. An orbital having a lobe on the axis will move to a higher energy level. It means the energy of eg levels is higher as compared to t2g levels.
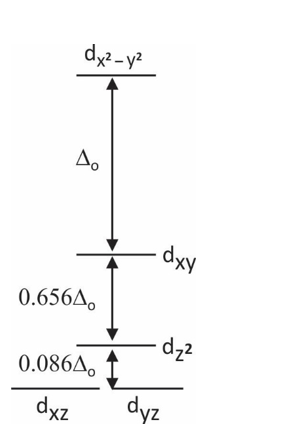
Tetrahedral Complexes:
In tetrahedral complexes, four ligands are present around the central atom. The splitting of d orbital takes place into two levels:
Top three energy levels (t2g) – dxy, dyz, dxz
Bottom two energy levels (eg) – dx2–y2, dz2
The reason behind this splitting is due to poor overlapping of the orbital between metal and ligand. In this, orbitals are directed on the axes, but ligands are not.
Square Planar Complexes:
In square planar complexes, four ligands are present around the central atom. In this, electrons of ligands get attracted towards the XY plane only. The four different energy levels for this complex are:
dx2–y2, dz2, dxy and both dyz, dxz.
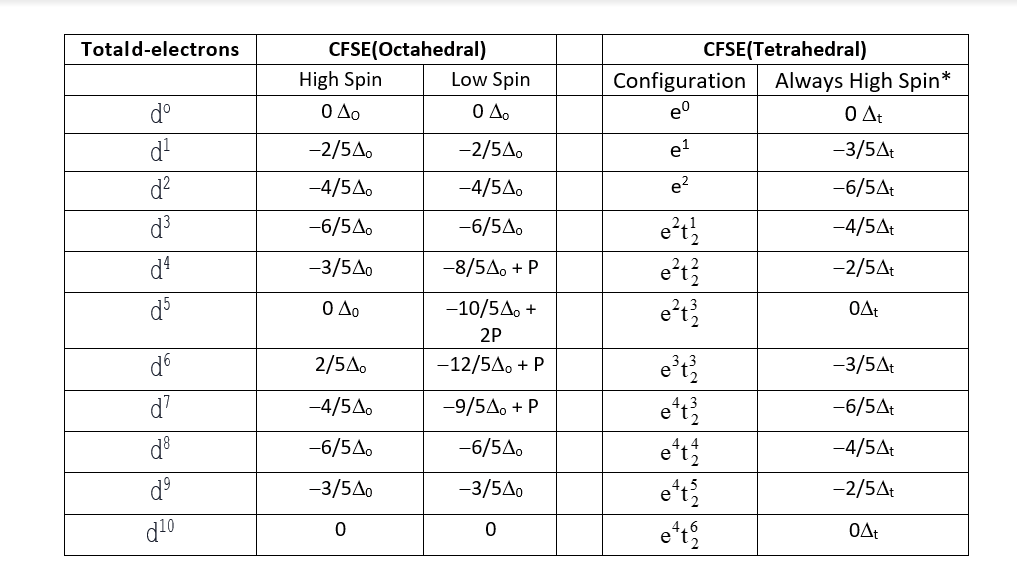
Crystal Field Stabilization Energy:
It is the difference between the ligand field energy and energy in an isotropic field. There are mainly four factors on which CFSE depends:
- Geometry
- Number of d electrons
- Spin pairing energy
- Nature of the ligand
The complete detail of the splitting of the d orbital along with the nature of the ligand in the both octahedral and tetrahedral environments is given below: