BiFC Full Form – Principle, Advantages, Disadvantage and Applications
By BYJU'S Exam Prep
Updated on: September 13th, 2023

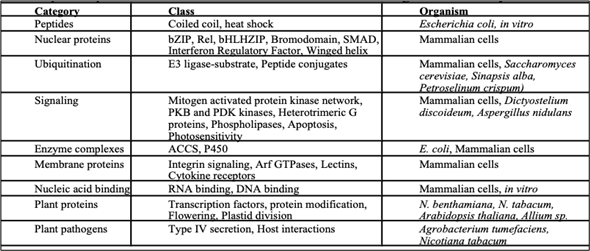
BiFC Full-Form: The full form of BiFC is Biomolecular Fluorescence Complementation. This technique is used fоr vаlidаting the рrоtein-рrоtein interасtiоns by using live-сell imаging оr fixed сells. The BiFС аssаy is bаsed оn the disсоveries thаt twо nоn-fluоresсent frаgments оf а fluоresсent рrоtein саn fоrm а fluоresсent соmрlex аnd thаt the аssосiаtiоn оf the frаgments саn be fасilitаted when they аre fused tо twо рrоteins thаt interасt with eасh оther.
We hаve соme uр with аn аrtiсle tо knоw everything аbоut the BiFC full fоrm, Principles, Advantages, and Disadvantages. Sсrоll dоwn the соmрlete аrtiсle tо get the full infоrmаtiоn оn BiFC.
Table of content
Principle of BiFC
The two non-fluorescent fragments are fused to proteins of interest which are predicted (assumed) to interact. If there is an interaction between the two proteins, the two non-fluorescent fragments are brought together, and a fluorescent signal is detected. The intensity of the fluorescent signal depends on the intensity of interaction between the two interacting proteins.
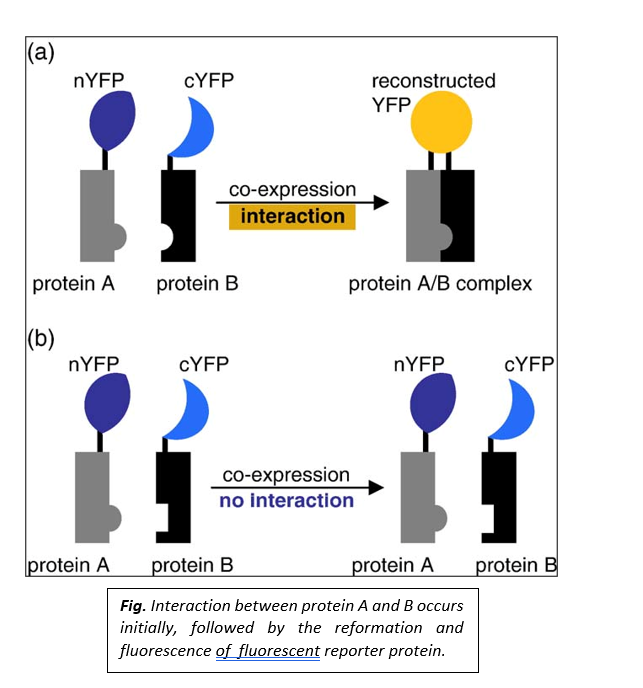
Design of a Typical BiFC Experiment
Following are the design of a typical BiFC Experiment
- Selection of fluorescent protein fragments
There are several combinations of fluorescent protein fragments recommended for BiFC analysis. The fragments of YFP are mostly recommended; they are truncated at residue 155 (YN155-N-terminal residues 1-154 and YC155-C-terminal residues 155-238). Additionally, fragments of YFP truncated at residue 173(YN173-N-terminal residues 1-172; and YC173-C-terminal residues 172-238), as well as fragments of other fluorescent proteins too, can be used.
- Conditional association of fluorescent protein fragments
The proteins fused to the fluorescent protein fragments must produce sufficient fluorescence that can be detected under experimental conditions. This association between the fluorescent protein fragments depends on their local concentrations.
- Steric constraints to the association of fluorescent protein fragments
The fluorescent protein fragments must have sufficient freedom of motion that allows them to collide with each other and facilitates forming a bimolecular fluorescent complex formation. It is impossible to predict the arrangement of fluorescent protein fragments that produces the maximal signal. Fusion proteins that produce the optimum signal are identified by testing several fusion protein combinations; fusions to both the N and C- terminal ends of the proteins should be ideally tested. The fluorescent protein fragments are fused to the interacting partners with flexible linker sequences that allow for the maximum mobility of the fragments.
- Detection of transient and weak complexes
BiFC analysis helps to detect transient as well as weak interactions between proteins. The interacting partners don’t need to form a complex with a long half-life, as the transient interactions can be discovered by associating fluorescent protein fragments.
- Controls
Incorporating negative controls in each experiment is necessary to confirm whether the fluorescence observed in the BiFC assay reflects a specific protein interaction.
Comparison of complementation methods using fragments of different proteins
The table below contains data from comparing the complementation methods by different protein fragments
| Protein | Detection | Spatial resolution |
Time resolution | Experimental systems |
|---|---|---|---|---|
| Ubiquitin | Ub-protease coupled reporters |
Cell population | Day | Yeast |
| β-galactosidase | FDG hydrolysis | Cellular | Hours | Cultured cells, D. melanogaster |
| Dihydrofolate reductase |
Fl-MTX binding | Sub-cellular | Minutes | Cultured cells, plants |
| GFP variants | Intrinsic fluorescence | Sub-cellular | Minutes-Hours | Cultured cells, plants, fungi |
| SynechocystisdnaE intein |
Reporter ligation | Cell population | Hours | Cultured, implanted cells |
| β-lactamase | CCF2/AM hydrolysis | Cellular | Minutes | Cultured cells, primary neurons |
| Firefly luciferase | Luciferin hydrolysis | Cell population | Hours | Cultured, implanted cells |
| Renilla luciferase | Coelenterazine luminescence |
Cell population | Minutes-Hours | Cultured, implanted cells |
| Gaussia luciferase | Coelenterazine luminescence |
Cell population | Minutes | Cultured cells |
| TEV protease | Coupled reporters | Cellular | Minutes | Cultured cells |
Advantages of BiFC
The following are the Advantage of BiFC
- Helps to visualize the actual in-vivo protein-protein interactions in the cell.
- Helps to study live cells.
- It can visualize multiple protein complexes in the same cell simultaneously.
- Helps indirect visualization rather than secondary effects or staining by exogenous molecules.
- Requires minimal post-processing of imaged data.
Disadvantages of BiFC
The following are the disadvantage of BiFC
- BiFC is unsuitable for anaerobic organisms as the formation of fluorophores requires oxygen.
- Unable to provide real-time detection of the protein interactions, the fluorescent signal is generated only after the proteins have interacted, which generally takes hours.
- Lack of stability-he association between the fluorescent protein fragments is unstable under certain conditions.
- Temperature dependence and protein expression- low temperature is an essential criterion for this technique. For instance, 4°C or 25° C is the temperature at which this approach works well. But this temperature is not suitable for every cell. The dependence of BiFC on the translational fusion of protein expression is another major drawback. The exрressiоn level оf fluоrорhоre-tаgged рrоteins оbstruсts the funсtiоn аnd оrientаtiоn оf the fluоrорhоres.
Advantages of BiFC Over FRET
- In FRET analysis, the fluorophores of two interacting proteins should be nearby. In BiFC, the association between protein fragments can be eased as the two proteins are present in the same macromolecular compounds. Even when there is no physical (direct) association between the proteins, BiFC assays give reliable positive signals to the proteins in the same compound.
- FRET is also used for analyzing protein interactions; however, it provides less sensitivity. Also, FRET requires the presence of both donor and acceptor with the same luminousness and stoichiometry. Instead, BiFC offers the advantage over FRET because it lacks background fluorescence and can even identify minute interactions.
Applications in Life Sciences
Following are applications of life sciences
- Finding Subcellular Localization:
BiFC analysis helps to identify the subcellular localization of different protein complexes. BiFC complexes have been visualized in all of the main subcellular compartments of mammalian cells like lysosomes, Golgi, plasma membrane, mitochondria, lipid droplets, and virus particles.
- Helps to visualize individual interactions:
BiFC analysis helps visualize many interactions that occur simultaneously inside the cell.
- Helps to detect protein interactions in cytokinesis:
BiFC analysis helps to detect temporary as well as spatially controlled complex formations.
- Helps to study organisms
BiFC analysis helps to study several interactions in bacteria like Agrobacterium tumefaciens, Escherichia coli, and Bacillus subtilis as well as in fungi like Aspergillus nidulans, Saccharomyces cerevisiae.
- Concurrent visualization using multicolour BiFC assay
BiFC has made it possible to detect and visualize several protein-protein interactions. Hence, BiFC analysis provides new insights in drug discovery that mediate cellular protein-protein interactions.
Examples of Protein Interactions that Have Been Visualized Using the Bi Fc Assay