BF3 is planar and electron deficient compound. Hybridization and number of electrons around the central atom, respectively are : (a). sp² and 8 (b). sp³ and 4 (c). sp³ and 6 (d). sp² and 6
By BYJU'S Exam Prep
Updated on: September 13th, 2023
We know that
BF3 has a boron atom with three outer-shell electrons in its ground state and three fluorine atoms containing seven outer electrons. If we observe further closely, one boron electron is unpaired in the ground state. During the formation, 2s orbital and two 2p orbitals hybridize. One of the empty p-orbital is left behind as the lone pair.
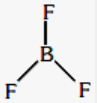
- The number of electrons around the boron atom is 6.
- sp2 is the hybridization of B
- It is of trigonal planar shape.
|
Name |
Boron Trifluoride |
|
Molecular Formula |
BF3 |
|
Type of Hybridization |
sp2 |
|
Angle of Bond |
120° |
|
Geometry |
Trigonal Planar |
Therefore, the hybridization and number of electrons around the central atom, respectively are sp2 and 6.
Summary:
BF3 is a planar and electron-deficient compound. Hybridization and the number of electrons around the central atom, respectively are : (a). sp2 and 8 (b). sp3 and 4 (c). sp3 and 6 (d). sp2 and 6
BF3 is a planar and electron-deficient compound. Hybridization and the number of electrons around the central atom, respectively are sp2 and 6.
Related Questions:-
- The Electron Concentration In An N Type Semiconductor Is The Same As The Hole Concentration In A P Type Semiconductor?
- Easiest Way To Remember The First 20 Elements Of A Periodic Table?
- A Cup Of Coffee Cools From 90 Degree Celcius To 80 Degree Celcius In T Minutes When The Room Temperature Is 20 Degree Celcius?


