- Home/
- CDS & Defence/
- Article
The number of primary alcohols possible with the formula C4H10O is – (A) 2 (B) 3 (C) 4 (D) 5
By BYJU'S Exam Prep
Updated on: September 25th, 2023
The number of primary alcohols possible with the formula C4H10O is 4. The alcohols possible with the molecular formula C4H10O is
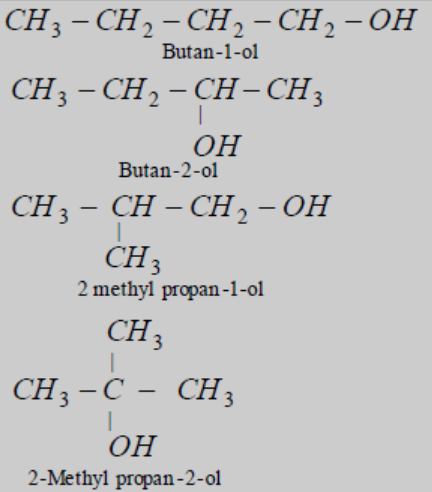
Table of content
Alcohols
Alcohols are organic compounds characterized by the presence of one, two, or more hydroxyl (-OH) groups attached to carbon atoms of an alkyl group or hydrocarbon chain. Alcohol is consumed as a beverage if it consists of 30-40% ethanol.
Types of Alcohols
- Primary alcohols
These are alcohols in which the OH group carbon atom is attached to a single alkyl group. A few examples of primary alcohols are methanol and ethanol. Two alkyl groups present may be structurally the same or different.
- Secondary Alcohols
In these alcohols the hydroxyl group carbon atom is attached on either side to two alkyl groups. Two alkyl groups available may be structurally the same or different.
- Tertiary alcohols
These alcohols contain hydroxyl group on the carbon atom attached to the 3-alkyl group. The alcohols physical properties mainly is based on the structure.
Applications of Alcohols
- It is used as antifreeze in mixtures of ethylene glycol solutions dissolved in water.
- Ethanol is an alcohol which is used as a preservative.
- Few alcohols are used in internal combustion engines as fuels, such as methanol.
- In medicine, some of them are used as preservatives in laboratory samples.
Summary:
The number of primary alcohols possible with the formula C4H10O is – (A) 2 (B) 3 (C) 4 (D) 5
The number of primary alcohols possible with the formula C4H10O is 4. Alcohols are organic compounds characterized by the presence of one, two, or more hydroxyl (-OH) groups attached to carbon atoms of an alkyl group or hydrocarbon chain.
