- Home/
- CDS & Defence/
- Article
The Formal Charge and P-O bond Order in PO4 3- Respectively are (A) 0.6, -0.75 (B) -0.75, 1.25 (C) 1.0, -0.75 (D) 1.25, -3
By BYJU'S Exam Prep
Updated on: September 25th, 2023
The formal charge and P-O bond order in PO4 3- respectively are -0.75 and 1.25. To calculate the formal charge in chemistry subject, students can use the following formula
Formal Charge [Number of valence electrons on atom] [non-bonded electrons number of bonds].
Formal change:
- The formal change of an atom (polyatomic) in a molecule or ion is computed by subtracting the number of valence electrons that are assigned to that atom in its lewis structure from the number of electrons that are present in its free state.
Bond order:
- Bond length and bond order are inversely related.
- A strong link between two atoms has a minimum length.
- While the maximum bond length is found in atoms with weak bonds.
- The Bond order formula is as follows:
Bond order = Number of bonds/ Number of resonating structures.
Table of content
Formal Charge and P-O bond Order in PO4 3- as follows
Phosphate ion:
- It is the salt of Phosphoric acid (H3 PO4).
- It is made up of one core P atom that is encircled by four O atoms in a tetrahedral configuration.
- The formal charge of the phosphate ion is negative three.
- The phosphate ion’s resonance structure is as follows:
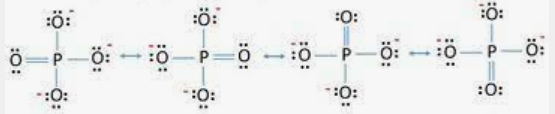
Let’s consider the lewis structure of PO4 3-:
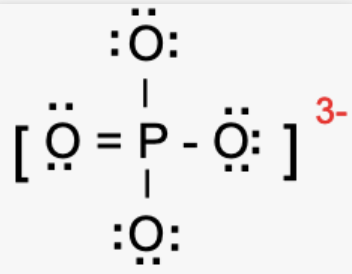
The atoms are marked as 1, 2, 3, and 4. Using the above formula, the formal charge on :
O atom that forms the double bond with the P atom,
Formal Charge = 6- 4 – ½(4) = 0
O atom that forms a single bond with P atom,
Formal Charge = 6 – 6 – ½(2) = 0
The central P atom,
Formal Charge = 5 – 0 – ½(10) = 0
Hence, we represent the Phosphate ion (PO4 3-) along with the formal charges as follows:
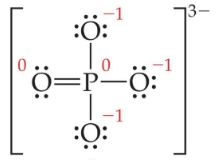
The formal charge on Perchloric acid (HClO4 ) is:
F.C = Total -ve charge/ Total number of oxygen atoms
F.C = -1-1-1 /4 = -¾ = -0.75
The Bond order on Perchloric acid (HClO4 ) is:
Bond order = Number of bonds/ Number of resonating structures
Bond order = 5/4 = 1.25 (no: of resonating structures =5)
Therefore, the formal charge and P-O bond order in PO4 3- respectively are -0.75 and 1.25.
Summary:
The Formal Charge and P-O bond Order in PO4 3- Respectively are (A) 0.6, -0.75, (B ) -0.75, 1.25, (C) 1.0, -0.75 (D) 1.25, -3
The formal charge and P-O bond orders in PO4 3- respectively are -0.75 and 1.25. To understand this in detail, students can use the formula and phosphate ion’s resonance structure like above.
