- Home/
- CDS & Defence/
- Article
How would you distinguish between primary, secondary, and tertiary alcohols?
By BYJU'S Exam Prep
Updated on: September 25th, 2023
According to the Lucas test, tertiary alcohol forms turbidity right away, secondary alcohols take longer to do so, and primary alcohol does not. When one, two, or more hydroxyl groups (OH) are bonded to the carbon atom in an alkyl group or hydrocarbon chain, the resultant organic molecule is known as alcohol.
Primary, Secondary, and Tertiary Alcohols
Primary alcohol:-
These alcohols have an alkyl group attached to the carbon that the hydroxide group is linked to, which is carbon 10C in these compounds.
Example: Ethanol (CH3 – CH2 – OH)
Secondary alcohol:-
These alcohols have two alkyl groups attached to the 20C carbon that the hydroxide group is bonded to.
Example: Propna-2-ol
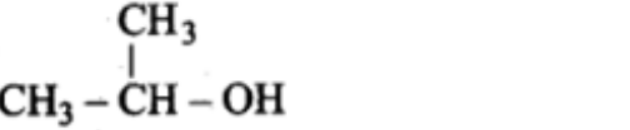
Tertiary alcohol:-
These are the alcohols in which the hydroxide group is coupled to a carbon atom with a 30C carbon atom that has three alkyl groups attached.
Example: 2-methylpropna-2-ol

Distinguishing between alcohols:
- To discriminate between primary (10C), secondary (20C), and tertiary (30C) alcohols, one uses the Lucas test.
- The reagent used in this case is known as the Lucas reagent and consists of anhydrous zinc chloride (ZnCl2) and hydrochloric acid.
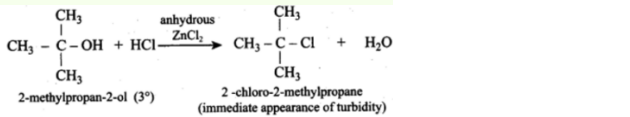
- When tertiary 30C alcohol is treated with this Lucas reagent, a turbidity forms practically immediately:
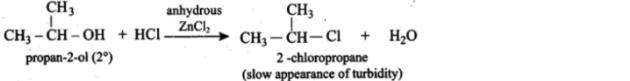
- They gradually create turbidity when secondary 20C alcohol is treated with this Lucas reagent:
- They don’t produce turbidity when primary 10C alcohol is treated with this Lucas reagent.
- The alcohol only becomes turbid after being heated.

As a result, primary alcohols do not produce turbidity, secondary alcohols do so slowly, and tertiary alcohols respond to the Lucas test by producing turbidity right away.
Summary:
How would you distinguish between primary, secondary, and tertiary alcohols?
According to the Lucas test, primary alcohols do not produce turbidity, whereas secondary alcohols do so slowly. Tertiary alcohols respond by producing turbidity right away. The presence of one, two, or more hydroxyl groups (OH) connected to the carbon atom in an alkyl group or hydrocarbon chain is what distinguishes alcohols from other organic molecules.
