- Home/
- CDS & Defence/
- Article
(i) Write the electron dot structure for sodium and oxygen. (ii) Show the formation of Na2O and MgO by electron transfer of electrons. (iii) What are the ions present in these compounds?
By BYJU'S Exam Prep
Updated on: September 25th, 2023
(i) The electron dot structure for sodium and oxygen is mentioned below.
(ii) The formation of Na2O and MgO by electron transfer of electrons is shown above.
(iii) The ions present in sodium oxide are 2Na+ and O2- and in Magnesium oxide are Mg2+ and O2-
Electron dot structure
- An electron dot structure illustrates how atoms in a molecule are chemically bonded to one another. They also display the overall number of lone pairs present
- Sodium has an atomic number = 11, and its electronic configuration = 1s22s22p63s1 hence, valence electron = 1
- Oxygen has an atomic number = 8, and its electronic configuration = 1s22s22p4 hence valence electron = 6
- We obtain the atom’s Lewis dot structure by positioning the valence electrons around it.
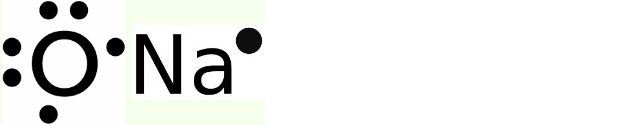
Formation of Na2O and MgO
- The electrical configuration of sodium with an atomic number of 11 is present in sodium oxide is 1s22s22p63s1, hence its valence electron = 1
- Electronic configuration of Oxygen with atomic number = 8 is 1s22s22p4, hence its valence electrons = 6
- Sodium just has one extra electron, whereas oxygen needs two to be stable. As a result, oxygen can acquire two electrons if two sodium atoms share one electron.
- By giving up two electrons, the two sodium atoms create sodium ions (2Na+). The oxygen atom receives two additional electrons to form an oxide ion (O2-).
- As a result, the oxygen octet is finished.

Ionic Compounds:
- Ionic compounds are produced when negatively charged anions combine with positively charged cations. Ionic compounds are electrically neutral.
- In Sodium oxide (Na2O) ions present are 2Na+ and O2-
- In Magnesium oxide (MgO) ions present are Mg2+ and O2-
Summary:
(i) Write the electron dot structure for sodium and oxygen. (ii) Show the formation of Na2O and MgO by electron transfer of electrons. (iii) What are the ions present in these compounds?
(I) As was already explained, sodium and oxygen have an electron dot structure.
(ii) The diagram above illustrates how electron transfer results in the creation of Na2O and MgO.
(iii) The ions present in sodium oxide are 2Na+ and O2- and in Magnesium oxide are Mg2+ and O2-
