- Home/
- CDS & Defence/
- Article
An aqueous solution of borax is?
By BYJU'S Exam Prep
Updated on: September 25th, 2023
(A) Neutral
(B) Amphoteric
(C) Basic
(D) Acidic
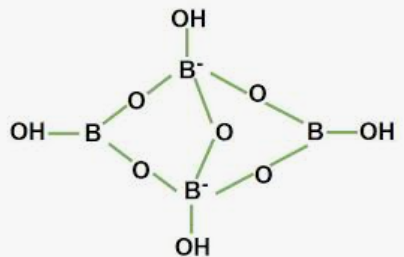
An aqueous solution of borax is basic. Sodium borate, sodium tetraborate, and disodium tetraborate are further names for borax. It is a white, powdered material. It contains boron, sodium, and oxygen in combination. Chemical formula: Na2 [B4 O5 (OH)4 ]·8H2O.
Table of content
Borax ionises when it is dissolved in water, and the hydrolysis of the Borate ion produces OH and Boric acid. In this case, Boric acid is a weak acid, while Na-OH is a strong alkali. As a result, the nature of the borax aqueous solution is alkaline.
Uses of Borax:
Herbicide, laundry enhancer, household cleaning, fungicide, etc.
An amphoteric substance in chemistry is a molecule or ion that reacts as both an acid and a base. In general, metallic oxides are basic, whereas non-metallic oxides are acidic. While some metallic or semi-metallic oxides are amphoteric, some non-metallic oxides are neutral. Al2 O3 and Ga2 O3 are examples of amphoteric compounds.
Summary:
An aqueous solution of borax is? (A) Neutral (B) Amphoteric (C) Basic (D) Acidic
Borax in an aqueous solution is basic. Borax also goes by the names sodium borate, sodium tetraborate, and disodium tetraborate. It is a powdery, white substance. Together, boron, sodium, and oxygen are present in it.
