Colloidal Solution: Definition, Types, Examples of Colloidal Solution
By BYJU'S Exam Prep
Updated on: November 14th, 2023
Colloidal Solution, also known as a colloidal suspension, refers to a mixture where finely divided particles are dispersed within a continuous medium. The particles in a colloidal solution are typically larger than individual molecules but smaller than those in a suspension. This heterogeneous mixture displays distinct properties and phenomena, such as the Tyndall effect, Brownian motion, and stability, making colloidal solutions an important topic of study in various scientific disciplines.
Colloidal Solutions find applications in a diverse range of fields, including medicine, industry, and environmental science. They are utilized in drug delivery systems, food production, cosmetics, and the manufacturing of paints, inks, and ceramics. Understanding the properties and behavior of colloidal solutions is crucial for comprehending their role in these applications and exploring their potential for innovative technological advancements.
Table of content
Colloidal Solution
Colloids are simply defined as a mixture in which one of the components has been broken down into minuscule particles spread throughout a second material. Colloidal particles are the name for tiny particles. Another way to put it is that colloids are solutions with solute particles ranging in size from 1 nm to 1000 nm. Colloids play a crucial role in various fields, including medicine, industry, and environmental science, due to their unique properties and wide range of applications.
Colloidal solutions, characterized by the presence of colloidal particles, display the Tyndall Effect where light is scattered upon interaction with the particles. These particles are small, typically ranging from 1 nanometer to 1 micrometer in size. Colloids exhibit a diverse nature, and according to the IUPAC, they are defined as substances in a state of subdivision where molecules or polymolecules particles with dimensions within the specified range are dispersed in a medium.
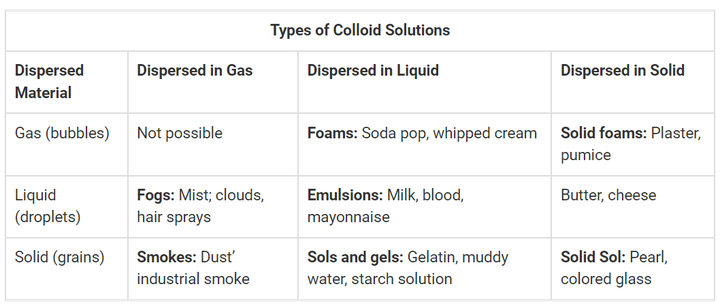
Types of Colloids
The dispersed substance and the phase it is disseminated in are two frequent criteria used to categories colloids. There are various types of colloids, such as:
- Sol: Sol is a liquid-based colloidal suspension of solid particles.
- Emulsion: In between two liquids is an emulsion.
- Foam: Foam is created when numerous gas particles are caught in a liquid or solid.
- Aerosol: Small liquid or solid particles are distributed in gas to create an aerosol.
A hydrocolloid is a colloidal system in which water serves as the dispersion medium. Depending on the amount of water available, the particles in the dispersed phase might go through various phases.
Colloidal Solution Examples
Not all mixtures are called colloids. Colloids are combinations where the suspended particles are evenly distributed throughout the other substance and do not settle at the button. These are a few illustrations of colloidal solutions:
- Perfume
- Blood
- Starch Solution
- Butter
- Cheese
- Dirty water
- Gelatin
- Paints
- Flame retardants
- Whipped cream
Properties of Colloidal Solution
Colloidal solutions exhibit various properties that distinguish them from other types of solutions. These properties can be categorized into colligative properties, physical properties, and mechanical properties. Following are the properties of colloidal solutions in detail below.
- Colligative Properties: The measured values of colligative properties, such as relative decrease in vapor pressure, increase in boiling point, decrease in freezing point, and osmotic pressure, are smaller than expected due to the presence of linked molecules and the low number of particles in the solution.
- Physical Properties: Colloidal solutions are heterogeneous in nature, consisting of two phases, the dispersion phase (particles) and the dispersion medium. These solutions are stable, constantly in motion, and do not settle at the bottom of a container. Colloidal particles can be filtered using common filter sheets.
- Mechanical Properties: Colloidal particles exhibit diffusion, moving from areas of higher concentration to lower concentration, albeit at a slower rate due to their larger size. Sedimentation occurs in colloidal solutions under the influence of gravity, with particles settling at a very slow rate, which is utilized in determining the molecular mass of macromolecules.
Purification of Colloidal Solutions
The colloidal solution can be purified using two basic methods: the condensation method (chemical procedures) and the dispersion method (physical techniques).
- Condensation Method: The following chemical processes are used in the condensation method to purify colloidal solution: hydrolysis, excessive cooling, oxidation, double decomposition, solvent exchange, change in physical state, etc.
- Dispersion Method: Bredig’s Arc Method, Electrical Dispersion, Mechanical Dispersion, and Peptization are the primary physical techniques used in the dispersion method to purify colloids.
Colloidal Solutions UPSC
Colloidal Solution refers to a heterogeneous mixture where finely divided particles are dispersed in a continuous medium. It is a topic covered under the UPSC Syllabus, falling under the section of General Science. Studying colloidal solutions is crucial as it enables candidates to grasp phenomena like the Tyndall effect, Brownian motion, and the distinct properties exhibited by colloids.
To gain a better understanding of the topic, candidates should study the types, examples, properties, and formation of colloidal solutions, as provided in the information above. Additionally, solving UPSC Previous Year Question Papers can help candidates familiarize themselves with the types of questions that may be asked in the exam, aiding in their exam preparation.
Colloidal Solutions UPSC Questions
Candidates are strongly advised to practice solving questions related to colloidal solution to enhance their understanding. By attempting the provided questions, candidates can gain a deeper comprehension of the core concepts and develop effective preparation strategies to excel in the exam.
Question: Which of the following properties is not characteristic of colloidal solutions? (A) Brownian motion (B) Sedimentation (C) Tyndall effect (D) Homogeneity
Answer: (B) Sedimentation
Question: Which of the following factors influences the stability of colloidal solutions? (A) Temperature (B) Concentration of the solute (C) pH of the solution (D) All of the above
Answer: (D) All of the above
Question for UPSC Mains: Discuss the applications and significance of colloidal solutions in various fields such as industry, medicine, and environmental science.
Question for UPSC Mains: Analyze the factors affecting the stability of colloidal solutions and explain how these factors can be manipulated to control the stability and properties of colloidal systems.
