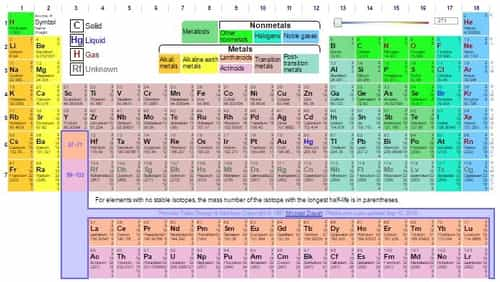
There are total 7 periods and 18 groups present in the Modern Periodic Table.
The S Block Element
The first two groups, s-block elements have chemical & Physical properties which are quite similar. Group 1 is known as alkali metals and group 2 known as Alkaline Earth metals.
Alkali Metals: Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Ru), Caesium (Cs), and Francium (Fr).
Mnemonic for Group 1: LiNa Kare Rub Cse Friyaad
Alkaline earth metals: It includes Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Br), and Radium (Ra).
Mnemonic for Group 2: Beta Mange Car Scooter Baap ram Ram.
The P Block Elements
The last six groups of the table consist of the P block element The groups are from 13 to 18. Non-metals, metalloids and poor metals are present in this group.
Also known as Boron group, it includes Boron (B), Aluminium (Al), Gallium (Ga), Indium (In), and Thallium (Tl).
Group 13: B A G I T.
Buffalo And Goat In Tamil
Group 14 :known as Carbon group or the group of Crystallogens, Tetragens or Tetrels. It includes Carbon (C), Silicon (Si), Germanium (Ge), Tin (Sn), and Lead (Pb).
Chauhan Sir Gives Standard Problems.
Group 15: known as the Nitrogen group. It includes Nitrogen (N), Phosphorus (P), Arsenic (As), Antimony (Sb), and Bismuth (Bi).
Nath Prasad Aur Sab Bhikari
Group 16:- of the periodic table is known as the group of of Chalcogens or Oxygen group. It includes Oxygen (O), Sulphur (S), Selenium (Se), Tellurium (Te), and the radioactive element Polonium (Po).
Omi Shukla Se TePo
Group 17: is known as the group of Halogens. It consist of Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), and Astatine (At).
Fir Call kar Bahaar AayI Aunty.
Group 18 is known as the group of Noble gases, excluding Helium. Normally, they are all odorless and colorless gases with very low chemical reactivity. The group includes Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), and the radioactive Radon (Rn).
Mnemonic for Group 18: He Never Arrived, Kriti Xero Run pe out.
Known as the Transition metals Groups 3 to 11 consist of all transition elements. Group 12 elements, which have its d as the subshell which are completely filled, are also known as the post-transition elements.
D-block elements and F-block elements show some similar properties throughout the period.
We can memorize these elements across the periods:
Period 4 elements are quite stable and many of them are very common in earth’s crust or core or both. D-block elements it includes are Scandium (Sc), Titanium (Ti), Vanadium (V), Chromium (Cr), Manganese (Mn), Iron (Fe), Cobalt (Co), Nickel (Ni), Copper (Cu) and Zinc (Zn).
Period 4: Science Ti(ea)cher Vandana Crore Mange Feko (FeCo) Ni Kyun(Cu) ki Zamaana hai
"Science Teacher Vandana Crore Mange Feko nahi kuki zamana hai"
Period 5 elements are known to fill their 5s shell first, then 4d shells and then 5p shells, with rhodium being the exception. The elements of this period show many exceptions to Maledung rule. D-block elements it includes are Yttrium (Y), Zirconium (Zr), Niobium (Nb), Molybdenum (Mo), Technetium (Tc), Ruthenium (Ru), Rhodium (Rh), Pd (Palladium), Silver (Ag) and Cadmium (Cd).
Period 5: Yaro Zarre Nabila bana Mohabaat mein T(c)eri, Ruke(Ru) Raho(Rh) Padmini(Pd) Aage(Ag) ise mili Coding(Cd)
"Yaro Zarre Nabila Bana Mohabbat Mein Teri Ruke raho PAdmini aage ise mili coding"
Period 6: Lanthanides are found in this. There are many elements in this group which are costly like gold. It includes Lutetium (Lu), Hafnium (Hf), Tantalum (Ta), Tungsten (W), Rhenium (Re), Osmium (Os), Iridium (Ir), Platinum (Pt), Gold (Au) and Mercury (Hg).
Period 6: L(u)a HafTa Warna Reh Us(Os) Irritating Popat ke saath Aur Hoj(g)a pagal.
"La Hafta Warna Reh Us Irritating Popat ke saath Aur Hoja pagal".
7th period of the modern Periodic Table consist of the radioactive elements. Including the actinides which consist of the heaviest naturally occurring element Californium. All other elements are synthesized artificially. D-block elements
it includes are Actinium (Ac), Rutherfordium (Rf), Dubnium (Db), Seaborgium (Sg), Bohrium (Bh), Hassium (Hs), Meitnerium (Mt), and Darmstadtium (Ds). Period 7: Ak(c)ele R(f)amu D(b)ang S(g)harma ko B(h)ook naH(s)i Machti Divas
"Akele Ramu Dabang Sharma ko Bhook nahi Machti Divas"
F-Block Elements
They are also known as inner transition elements. They can be divided into Lanthanides (also known as rare earth elements) and Actinides.
Lanthanides include elements which are: Cerium (Ce), Praseodymium (Pr), Neodymium (Nd), Promethium (Pm), Samarium (Sm), Europium (Eu), Gadolinium (Gd), Terbium (Tb), Dysprosium (Dy), Holmium (Ho), Erbium (Er), Thulium (Tm), Ytterbium (Yb) and Lutetium (Lu).
We can learn all these in three parts:
Cerium (Ce), Praseodymium (Pr), Neodymium (Nd), Promethium (Pm), and Samarium (Sm)
Celina and Paro Ne dande se Pammy aur Simmy ko mara.
Europium (Eu), Gadolinium (Gd), Terbium (Tb), Dysprosium (Dy), and Holmium (Ho)
"Europe Gayi TaBu aur Diwani Ho gayi"
Erbium (Er), Thulium (Tm), Ytterbium (Yb) and Lutetium (Lu)
"E re, dekh Tamatar Yellow aur blue hain"
There are also some other elements which are not so important but at many times in state-level exam the atomic mass and number can be asked.
1.Thorium (Th), Protactinium (Pa), Uranium (U), and Neptunium (Np)
Theory Padhle Ullu No problem
2.Plutonium (Pu), Americium (Am), Curium (Cm), Berkelium (Bk)
Purane Aam K(C)am Bekar.

NDA I 2022 Exam Courses- Click Here
Thanks
Download the BYJU’S Exam Prep App Now
The Most Comprehensive Exam Prep App
#DreamStriveSucceed





Comments
write a comment