There are total 7 periods and 18 groups present in the Modern Periodic Table.
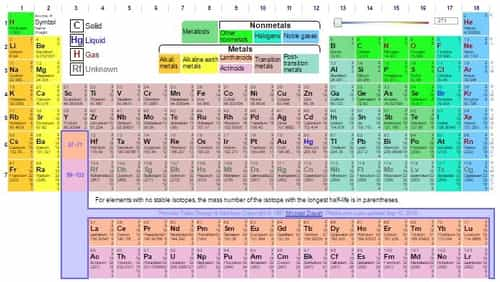
Properties of Periodic Table
- There are 18 vertical columns or groups. These are further sub-divided into A and B (groups I to VII), VIII group and zero group.
- Member of the same group have the similar electronic configuration of the valence shell and thus show the same valency.
- Elements of groups IA to VIIA are called groups of typical elements, representative elements or normal elements.
- Groups IA and IIA are strongly metallic and are called group of 'alkali metals and alkaline earth metals'.
- Groups IB to VII B and VIII lie in the middle of the table between IIA and IIIA groups and are called groups of transition elements. They consist of metals.
- The Zero group consists of 'Noble gases'.
- There are 7 horizontal rows in the periodic table. These are called the periods.
- In a period, the number of valence shell remains the same for all elements. However, the number of electrons in the valence shell increases from left to right.
- The number of elements present in each period is given in the following table.
- The 6th period consists of elements that have atomic numbers 58 to 71. They are called Lanthanides. The 7th period consists of elements that have atomic numbers 90 to 105. They are called Actinides. Both of them are called inner transition elements.
- The 7th period is an incomplete period as it has only 23 elements.
- Lanthanides and actinides are not accommodated in the main body of the periodic table but are placed separately at the bottom of the table.
- The position of hydrogen is not certain. Thus it can be placed in both group IA and group VIIA.
- Group VIIA elements are called halogens or salt producers.
Tricks to Remember Modern Periodic Table
The S Block Element
The first two groups, s-block elements have chemical & Physical properties which are quite similar. Group 1 is known as alkali metals and group 2 known as Alkaline Earth metals.
Alkali Metals: Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Ru), Caesium (Cs), and Francium (Fr).
Mnemonic for Group 1: LiNa Kare Rub Cse Friyaad
Alkaline earth metals: It includes Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Br), and Radium (Ra).
Mnemonic for Group 2: Beta Mange Car Scooter Baap ram Ram.
The P Block Elements
The last six groups of the table consist of the P block element The groups are from 13 to 18. Non-metals, metalloids and poor metals are present in this group.
Also known as Boron group, it includes Boron (B), Aluminium (Al), Gallium (Ga), Indium (In), and Thallium (Tl).
Group 13: B A G I T.
Buffalo And Goat In Tamil
Group 14 :known as Carbon group or the group of Crystallogens, Tetragens or Tetrels. It includes Carbon (C), Silicon (Si), Germanium (Ge), Tin (Sn), and Lead (Pb).
Chauhan Sir Gives Standard Problems.
Group 15: known as the Nitrogen group. It includes Nitrogen (N), Phosphorus (P), Arsenic (As), Antimony (Sb), and Bismuth (Bi).
Nath Prasad Aur Sab Bhikari
Group 16:- of the periodic table is known as the group of of Chalcogens or Oxygen group. It includes Oxygen (O), Sulphur (S), Selenium (Se), Tellurium (Te), and the radioactive element Polonium (Po).
Omi Shukla Se TePo
Group 17: is known as the group of Halogens. It consist of Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), and Astatine (At).
Fir Call kar Bahaar AayI Aunty.
Group 18 is known as the group of Noble gases, excluding Helium. Normally, they are all odorless and colorless gases with very low chemical reactivity. The group includes Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), and the radioactive Radon (Rn).
Mnemonic for Group 18: He Never Arrived, Kriti Xero Run pe out.
Known as the Transition metals Groups 3 to 11 consist of all transition elements. Group 12 elements, which have its d as the subshell which are completely filled, are also known as the post-transition elements.
D-block elements and F-block elements show some similar properties throughout the period.
We can memorize these elements across the periods:
Period 4 elements are quite stable and many of them are very common in earth’s crust or core or both. D-block elements it includes are Scandium (Sc), Titanium (Ti), Vanadium (V), Chromium (Cr), Manganese (Mn), Iron (Fe), Cobalt (Co), Nickel (Ni), Copper (Cu) and Zinc (Zn).
Period 4: Science Ti(ea)cher Vandana Crore Mange Feko (FeCo) Ni Kyun(Cu) ki Zamaana hai
"Science Teacher Vandana Crore Mange Feko nahi kuki zamana hai"
Period 5 elements are known to fill their 5s shell first, then 4d shells and then 5p shells, with rhodium being the exception. The elements of this period show many exceptions to Maledung rule. D-block elements it includes are Yttrium (Y), Zirconium (Zr), Niobium (Nb), Molybdenum (Mo), Technetium (Tc), Ruthenium (Ru), Rhodium (Rh), Pd (Palladium), Silver (Ag) and Cadmium (Cd).
Period 5: Yaro Zarre Nabila bana Mohabaat mein T(c)eri, Ruke(Ru) Raho(Rh) Padmini(Pd) Aage(Ag) ise mili Coding(Cd)
"Yaro Zarre Nabila Bana Mohabbat Mein Teri Ruke raho PAdmini aage ise mili coding"
Period 6: Lanthanides are found in this. There are many elements in this group which are costly like gold. It includes Lutetium (Lu), Hafnium (Hf), Tantalum (Ta), Tungsten (W), Rhenium (Re), Osmium (Os), Iridium (Ir), Platinum (Pt), Gold (Au) and Mercury (Hg).
Period 6: L(u)a HafTa Warna Reh Us(Os) Irritating Popat ke saath Aur Hoj(g)a pagal.
"La Hafta Warna Reh Us Irritating Popat ke saath Aur Hoja pagal".
7th period of the modern Periodic Table consist of the radioactive elements. Including the actinides which consist of the heaviest naturally occurring element Californium. All other elements are synthesized artificially. D-block elements
it includes are Actinium (Ac), Rutherfordium (Rf), Dubnium (Db), Seaborgium (Sg), Bohrium (Bh), Hassium (Hs), Meitnerium (Mt), and Darmstadtium (Ds). Period 7: Ak(c)ele R(f)amu D(b)ang S(g)harma ko B(h)ook naH(s)i Machti Divas
"Akele Ramu Dabang Sharma ko Bhook nahi Machti Divas"
F-Block Elements
They are also known as inner transition elements. They can be divided into Lanthanides (also known as rare earth elements) and Actinides.
Lanthanides include elements which are: Cerium (Ce), Praseodymium (Pr), Neodymium (Nd), Promethium (Pm), Samarium (Sm), Europium (Eu), Gadolinium (Gd), Terbium (Tb), Dysprosium (Dy), Holmium (Ho), Erbium (Er), Thulium (Tm), Ytterbium (Yb) and Lutetium (Lu).
We can learn all these in three parts:
Cerium (Ce), Praseodymium (Pr), Neodymium (Nd), Promethium (Pm), and Samarium (Sm)
Celina and Paro Ne dande se Pammy aur Simmy ko mara.
Europium (Eu), Gadolinium (Gd), Terbium (Tb), Dysprosium (Dy), and Holmium (Ho)
"Europe Gayi TaBu aur Diwani Ho gayi"
Erbium (Er), Thulium (Tm), Ytterbium (Yb) and Lutetium (Lu)
"E re, dekh Tamatar Yellow aur blue hain"
There are also some other elements which are not so important but at many times in state-level exam the atomic mass and number can be asked.
1.Thorium (Th), Protactinium (Pa), Uranium (U), and Neptunium (Np)
Theory Padhle Ullu No problem
2.Plutonium (Pu), Americium (Am), Curium (Cm), Berkelium (Bk)
Purane Aam K(C)am Bekar.
Periodic Table : PDF
More from us:
Important Study Notes for Defence Exams
Keep Learning




Comments
write a comment