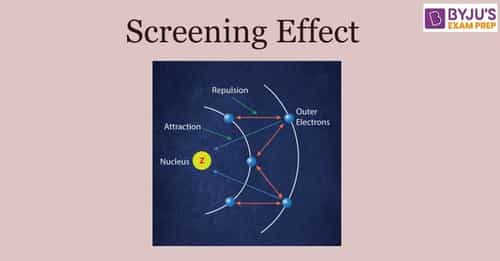
The Screening Effect is also called as shielding effect. It is a chemistry phenomenon that occurs when a nuclear lessens its force of attracting valence or the outer shell electrons due to the robust and vital force of inner shell electrons. As a result, the outer electrons experience attraction from the nucleus and repulsion from the electrons on the inner shell.
The shielding effect is a particular type of electric field screening described when the effective nuclear charge is lowered on the electron cloud. A summary of the screening effects is listed below:
Screening Effect with Examples
The Screening Effect explains the structure of electrons inside the nucleus. The closest shell is the s-shell, and electrons in this shell experience the most potent force from the nucleus. The second shell is the p-shell shielded or screened by electrons of the s-shell. Finally, the d-shell electrons are screened by the s-shell and p-shell both.
Nuclear fission is the best example of the Screening Effect. In this method, the electrons get pulled away from the middle of the atom to the farthest distance.

Define Screening Effect
All atoms consist of the nucleus in the center, around which all the electrons revolve in various orbits. The electrons present in the outer and inner shells are strongly attracted to the nucleus in the center. An atom's outer or outermost electron is termed the "valence electron".
The valence electrons experience an electrostatic force of attraction by the protons that reside in the nucleus center. In contrast, they also experience a strong force of repulsion from the inner shell electrons due to similarity in charges (+). The screening effect arises when the attractive force of the nucleus becomes less as compared to the repulsive force by the inner electrons.
☛ Also Check: Current Affairs Today
Overview and Order of Screening Effect
Below are the important features of the screening effect in chemistry. Get to know why the screening effect occurs, the order of screening effect, when the screening effect increases ionization energy, and many more such important concerns.
- The broader the electron shells in the space, the weaker the electrons' interaction between the nucleus and the electron.
- The higher the nuclear force of attractions, the greater the energy required to eject an electron from an isolated gaseous atom, also termed ionization enthalpy.
- The screening effect influences ionization enthalpy and is inversely proportional to it.
- The formula for screening effect in the electron shells is: s>p>d>f
Screening Effect for Alkali Metals
In the case of Alkali metals (any of the elements found in Group IA of the periodic table), the outer ns-electron is shielded by the interior electrons, due to which the effective charge on the nucleus is small.
As we move down the group in the periodic table, the effect of increasing protons is less than the shielding effect. Only one valence electron is required to be lost to complete the octet configuration. The ionization energy of Alkalis decreases down the group, and hence the valence electrons are lost quickly. These metals reducing power increases down the group, which is why they act as good reducing agents (chemical species donating electrons).
What is the Difference between Shielding and Screening Effect?
The screening effect is also known as the shielding effect. This phenomenon is basically the reduction in the effective charge of the nucleus on the electron cloud, which is seen due to dissimilarities n the attraction forces between the nucleus and electrons. Both the terms are somewhat the same, so there is no difference between shielding and screening effect.
FAQs on Screening Effect
What is the Screening Effect of orbitals?
Each atom has more than one electron shell that experiences a force of attraction or repulsion between the nucleus and an electron, called a screening effect. As the number of shells of electrons increases, more shielding effect is felt by the elections lying on the outermost shells.
What is an example of a screening effect?
One of the best examples of the screening effect is Nuclear Fission. This reaction occurs when a heavy nucleus splits into two smaller nuclei. The splitting of the nucleus produces gamma photons that emit an immense amount of energy.
The screening effect is not observed in which atom?
The Screening Effect is mainly seen by the elements having higher atomic numbers. The phenomenon arises due to the difference in the attraction force between the nucleus and more than one electron. The screening effect is not observed in atoms having only one electron in their shell. Some examples are He+, Li2+, and Be3+.
How strong is the screening effect of d electrons?
The screening effect of d electrons is lower than p-electrons as they are towards a more outer side than the p-shell. D and f electrons generally experience a poor screening effect than p and s-shell electrons. The shells more close to the nucleus experience more screening effect.
What is the screening effect order in any given atom shell?
In a given shell, the order of screening effect is s>p>d>f, where the s-shell possesses the highest shielding effect and the f-shell experiences the lowest shielding effect, depending upon the distance of the shells from the center of the nucleus.
What is the magnitude on which the screening effect depends?
The magnitude of the screening effect depends upon the presence of a total of inner electrons. The screening effect increases if the number of inner electrons in any atom increases.