Fuels
- The substance, which produces heat and light on combustion are called fuels.
- A strong foul-smelling substance, called ethyl mercaptan is added to LPG to detect its leakage as LPG is an odourless gas.
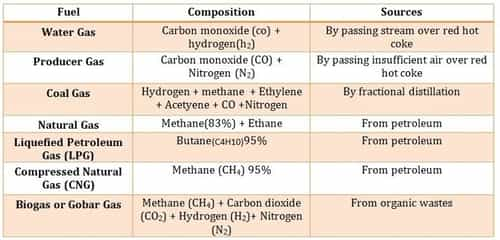
Some Important Fuels and their Compositions
Physical and Chemical Changes
- Physical changes change, which only affect the physical properties like colour, hardness, density, melting point etc. of matter, but do not affect the composition and chemical properties of matter.
- A physical change is temporary, while a chemical change is permanent.
- Crystallisation, sublimation, 'boiling, melting, vaporisation, cutting of trees, dissolving sugar or salt in water etc. are physical changes.
- Chemical changes affect the composition as well as chemical properties of matter and result in the formation of a new substance.
- Burning of fuel, burning of candle and paper, electrolysis of water, photosynthesis, the ripening of fruits etc, are examples of chemical changes
Coal
Coal is obtained by carbonization of vegetable matter and is available in different varieties:
- Peat- 60% C
- Lignite or Brown Coal – 70% C
- Bituminous – 60 to 80 % C
- Anthracite Coal – 90% C
- Fame
Flame contains three parts
- The innermost Part- which is black due to the presence of unburned carbon particles- has the lowest temperature.
- The middle part – is yellow due to incomplete combustion of fuel.
- The outermost part- which is blue due to the complete combustion of fuel is the hottest and is used by goldsmiths to heat the gold.
Fire Extinguishers
- Water extinguishes the fire because as it evaporates, the vapours surround the burning substance, cutting off the oxygen supply, and thus inhibiting the burning process.
- In the case of electrical or oil (petrol) fires, water cannot be used as an extinguisher. This is because water is a conductor of electricity and heavier than oil. Thus, oil floats over it and continues to burn.
- Carbon dioxide, which is generated by the reaction of baking soda with acid, is used to extinguish electrical or oil fires. The quality of petrol is measured in terms of octane number and that of diesel in terms of cetane number.
Thanks
Download the BYJU’S Exam Prep App Now.
The most comprehensive exam prep app
#DreamStriveSucceed






Comments
write a comment