Time Left - 25:00 mins
Previous Year Questions on Kinetic Theory of Gasses
Attempt now to get your rank among 704 students!
Question 1
Figure shows graphs of pressure versus density for an ideal gas at two temperatures T1 and T2.


Question 2
We have a jar A filled with gas characterized by parameters P, V and T and another jar B filled with gas with parameters 2P,  and 2T, where the symbols have their usual meanings. The ratio of the number of molecules of jar A to those of jar B is –
and 2T, where the symbols have their usual meanings. The ratio of the number of molecules of jar A to those of jar B is –
Question 3
A gas molecule of mass m is incident normally on the wall of the containing vessel with velocity u. After the collision, magnitude of the change in momentum of the molecule will be –
Question 4
Gas at pressure P0 is contained in a vessel. If the masses of all the molecules are halved and their speed doubled, the resulting pressure P will be equal to –
Question 5
If the total translational kinetic energy of H2 molecules is 7.5 × 103 J for the gas filled in a container of 10-liter capacity, then the pressure will be in Nm–2 -
Question 6
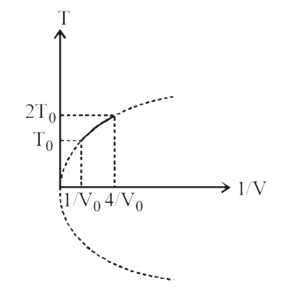
Figure shows a parabolic graph between T and  for a mixture of a gas undergoing an adiabatic process. What is the ratio of vrms and speed of sound in the mixture?
for a mixture of a gas undergoing an adiabatic process. What is the ratio of vrms and speed of sound in the mixture?
Question 7
A gas is found to obey the law P2V = constant. The initial temperature and volume are T0 and V0. If the gas expands to a volume 3V0, then the final temperature becomes
Question 8
A gas is found to obey the law P2V = constant. The initial temperature and volume are T0 and V0. If the gas expands to a volume 3V0, then the final temperature becomes
Question 9
One mole of ideal monoatomic gas (γ = 5/3) is mixed with one mole of diatomic gas (γ = 7/5). What is γ for the mixture? γ denotes the ratio of specific heat at constant pressure, to that at constant volume.
Question 10
Three perfect gases at absolute temperatures T1, T2 and T3 are mixed. The masses of molecules are m1, m2 and m3 and the number of molecules are n1, n2 and n3 respectively. Assuming no loss of energy, the final temperature of the mixture is
- 704 attempts
- 11 upvotes
- 44 comments
Tags :
Jun 15JEE & BITSAT

