Time Left - 25:00 mins
Previous Year Questions on Heat & Thermodynamics
Attempt now to get your rank among 818 students!
Question 1
Two spherical vessels of equal volume are connected by a narrow tube. The apparatus contains an ideal gas at one atmosphere and 300K. Now if one vessel is immersed in a bath of constant temperature 600K and the other in a bath of constant temperature 300K, then the common pressure will be –
Question 2
A piston shown in figure can move freely inside a non-conducting cylinder. One mole of an ideal gas (γ = 1.5) is in left chamber and right chamber is evacuated. Initially the piston is held at middle of cylinder and temperature of gas is 300K. If the piston is released suddenly, temperature of gas will become–


Question 3
The temperature at the bottom of a high waterfall is higher than that at the top because
Question 4
A system changes from the state (P1, V1) to (P2, V2) as shown in the figure below. What is the work done by the system –



Question 5
During an adiabatic expansion of 2 moles of a gas, the change in internal energy was found to be equal to –100J. The work done during the process by the gas will be equal to –
Question 6
An ideal gas is taken through series of changes ABCA. The amount of work involved in the cycle is –


Question 7
In the figure shown, one of the curves is for adiabatic process and one the curves is for isothermal process. The most probable curve for adiabatic process is given by the curve –


Question 8
Helium gas goes through a cycle ABCDA (consisting of two isochoric and two isobaric lines) as shown in figure. Efficiency of this cycle is nearly (Assume the gas to be close to ideal gas)
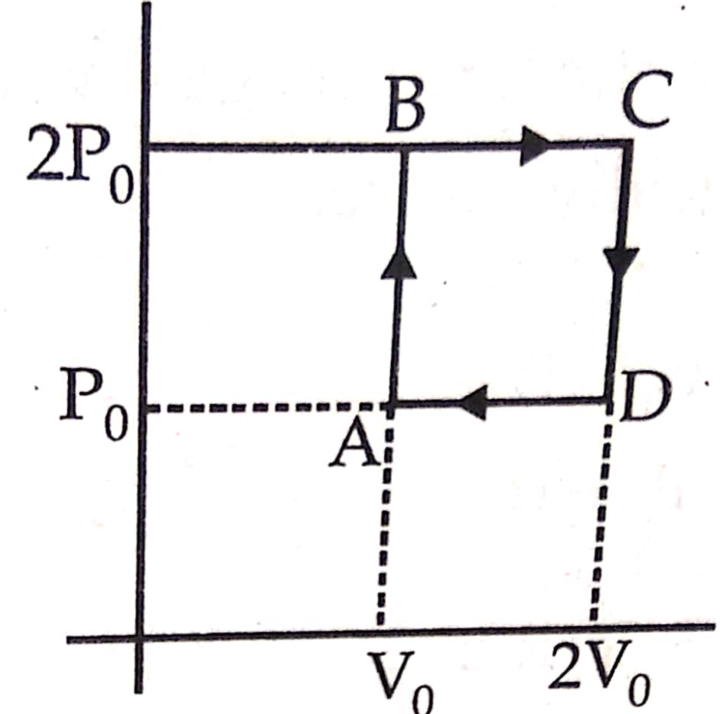
Question 9
A Carnot engine whose efficiency is 40%, takes in heat from a source maintained at a temperature of 500 K. It is desired to have an energy of efficiency 60%. Then, the intake temperature for the same exhaust (sink) temperature must be
Question 10
If Cp and Cv denote the specific heats of nitrogen per unit mass at constant pressure and constant volume respectively, then
- 818 attempts
- 14 upvotes
- 37 comments
Tags :
Mar 12JEE & BITSAT

