Time Left - 01:00:00 mins
JEE 2020: Weekly Quiz 13 (27.07.2019)
Attempt now to get your rank among 183 students!
Question 1
A rope of mass 5 kg is moving vertically in vertical position with an upwards force of 100 N acting at the upper end and a downwards force of 70 N acting at the lower end. The tension (in Newton) at midpoint of the rope is
Question 2
A particle of small mass m is joined to a very heavy body by a light string passing over a light pulley. Both bodies are free to move. The total downward force in the pulley is
Question 3
In the arrangement shown in the fig, the block of mass m = 2 kg lies on the wedge on mass M = 8 kg. Find the initial acceleration of the wedge if the surfaces are smooth and pulley & strings are massless.

Question 4
A monkey of mass 20 kg is holding a vertical rope. The rope can break when a mass of 25 kg is suspended from it. What is the maximum acceleration with which the monkey can climb up along the rope?
Question 5
Both the blocks shown here are of mass m and are moving with constant velocity in direction shown in a resistive medium which exerts equal constant force on both blocks in direction opposite to the velocity. The tension in the string connecting both of them will be (Neglect friction)

Question 6
A force exerts an impulse I on a particle changing its speed from u to 2u. The applied force and the initial velocity are oppositely directed along the same line. The work done by the force is
Question 7
A parallel beam of particles of mass m moving with velocity v impinges on a wall at an angle θ to its normal. The number of particles per unit volume in the beam is n. If the collision of particles with the wall is elastic, then the pressure exerted by this beam on the wall is
Question 8
A boy hits a baseball with a bat and imparts an impulse J to the ball. The boy hits the ball again with the same force, except that the ball and the bat are in contact for twice the amount of time as in the first hit. The new impulse equals.
Question 9
A system of two blocks A and B are connected by an inextensible massless string as shown. The pulley is massless and frictionless. Initially, the system is at rest when a bullet of mass ‘m’ moving with a velocity ‘u’ as shown hits the block ‘B’ and gets embedded into it. The impulse imparted by tension force to the block of mass 3 m is :
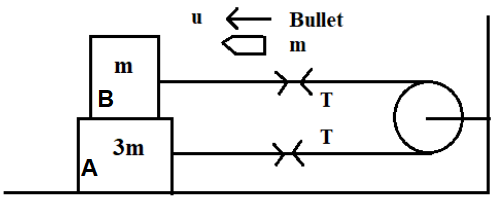

Question 10
In the figure (i), (ii) & (iii) shown the objects A, B & C are of same mass. String, spring & pulley are massless. C strikes B with velocity ‘u’ in each case and sticks to it. The ratio of velocity of B in case (i) to (ii) to (iii) is

Question 11
For which of the following values of m, the area of the region bounded by the curve y = x – x2 and the line y = mx equals 
Question 12
Area enclosed between the curve y2 (2a – x) = x3 and line x,= 2a above x-axis is
Question 13
If  and
and  , then the value of a and b will be respectively
, then the value of a and b will be respectively
Question 14
Question 15
Evaluate:
Question 16
The value of  depends on
depends on
Question 17
The values of ‘a’ for which  are
are
Question 18
The absolute value of  is
is
Question 19
Consider the integrals
 and
and  . The greatest of these integrals is
. The greatest of these integrals is
Question 20
If  , then
, then
 , then
, then Question 21
Which of the following statement(s) is/are correct?
(I) The removal of one electron from Na+(g) ion requires more energy than that from Mg+(g)
(II) The extent of hydration of Na+ ion is more than that of K+ ion.
(III) Ionic radii follows the order for three elements (X, Y, Z) of same period belonging to group 1, 2 and 3 (i.e. I A, II A and III A) in the periodic table is X+ > Y2+ > Z3+.
(IV) With the increasing electronegativity (which increases with increasing positive charge), the basic strength of any elemental oxide decreases
(I) The removal of one electron from Na+(g) ion requires more energy than that from Mg+(g)
(II) The extent of hydration of Na+ ion is more than that of K+ ion.
(III) Ionic radii follows the order for three elements (X, Y, Z) of same period belonging to group 1, 2 and 3 (i.e. I A, II A and III A) in the periodic table is X+ > Y2+ > Z3+.
(IV) With the increasing electronegativity (which increases with increasing positive charge), the basic strength of any elemental oxide decreases
Question 22
Identify the wrong statement among the following.
Question 23
Which one of the following arrangements does not give the correct picture of the trends indicated against it?
Question 24
Amongst the elements with following electronic configurations, which one of them may have the highest ionisation energy ?
Question 25
The formation of the oxide ion O2- (g), from oxygen atom requires first an exothermic and then an endothermic step as shown below,

Thus, process of formation of O2- in gas phase is unfavorable even though O2- is isoelectronic with neon. It is due to the fact that

Thus, process of formation of O2- in gas phase is unfavorable even though O2- is isoelectronic with neon. It is due to the fact that
Question 26
Fourteen elements of both 6th and 7th periods are called _____ and _____ respectively.
Question 27
The electronegativity values of the elements are useful in predicting :
Question 28
IUPAC name of the complex Na2[NiCl4] is-
Question 29
The IUPAC name for [Pt(py)4] [PtCl4] is
Question 30
The IUPAC name of [CoCl2(en)2]Cl is
- 183 attempts
- 4 upvotes
- 7 comments
Tags :
Dec 11JEE & BITSAT

