Time Left - 30:00 mins
Class XI Physics Thermodynamics 7
Attempt now to get your rank among 1141 students!
Question 1
What, if anything, is wrong with this refrigerator?


Question 2
Calculate the thermal efficiency of this heat engine?


Question 3
The temperature of steam coming from a boiler to a cylinder of a steam engine is 120 °C; the steam condenses in a cold reservoir with a temperature of 40 °C. What is the maximum work performed by the engine in ideal conditions with a heat consumption of 4.2 kJ?
Question 4
A refrigerator uses 400 J of work to remove 200 J of heat from its contents. How much heat must it reject to its surroundings?
Question 5
An ideal gas has initial volume V and pressure P. In doubling its volume the minimum work done will be in the following process (of given processes)
Question 6
Question are based on the following paragraph.
Two moles of helium gas are taken over the cycle ABCDA, as shown in the P–T diagram.

Two moles of helium gas are taken over the cycle ABCDA, as shown in the P–T diagram.
Assuming the gas to be ideal the work done on the gas in taking it from A to B is:
Question 7
Helium gas goes through a cycle ABCDA (consisting of two isochoric and two isobaric lines) as shown in figure. Efficiency of this cycle is nearly (Assume the gas to be close to ideal gas)
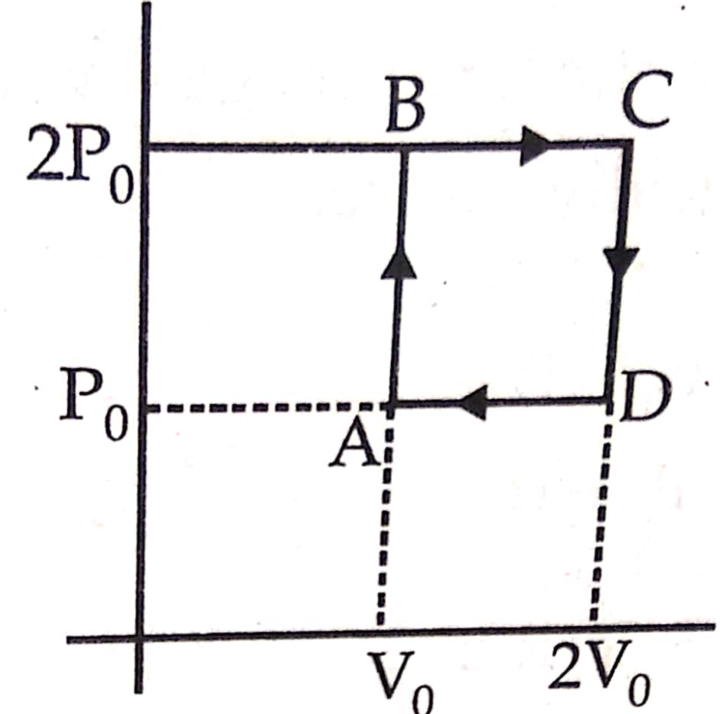
Question 8
Which of the following is incorrect regarding the first law of thermodynamics?
- 1141 attempts
- 12 upvotes
- 24 comments
Tags :
Mar 26JEE & BITSAT

