Time Left - 24:00 mins
CSIR NET 2022| Revision Booster Chemical Science|(23 Jan)
Attempt now to get your rank among 92 students!
Question 1
The complex with spin-only magnetic moment of 2.82BM is:
Question 2
Predict the suitable reagents to carry out the given reaction:

Question 3
At 100° C, the specific volumes of water and steam are, respectively 1 c.c. and 1673 c.c. respectively. Determine the change in vapor pressure (in mm Hg) of the system by 1°C change in temperature. The molar heat of vaporization of water in this range may be taken as 40584.8 J mol-1.
Question 4
Which statement is incorrect about the Lanthanide metals?
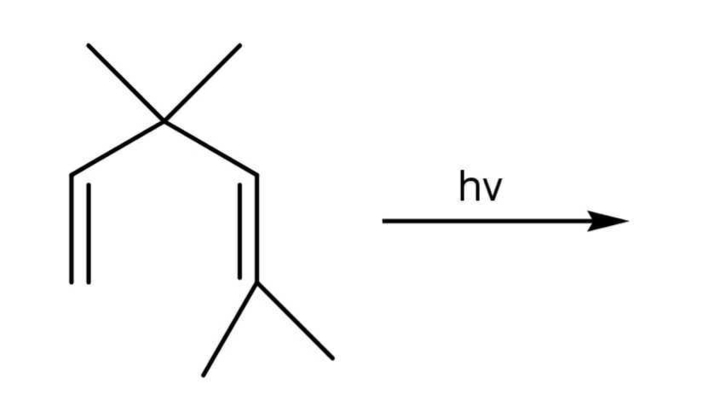
Question 5
Determine the major product formed in the reaction given below.
Question 6
Calculate the pressure exerted by one mole of carbon dioxide gas in a 1.32 dm3 vessel at 48°C using the van der Waals equation. The van der Waals constants are a=3.59 dm6 atm mol-2 and b=0.0427 dm3 mol-1.
Question 7
Determine the quantities that are held fixed in a canonical ensemble.
Question 8
The major product formed in the following reaction is

Question 9
Which among the following is incorrect?
Question 10
At 248°C, the Kp for the reaction,
SbCl5(g) ![]() SbCl3(g) + Cl2(g) is 1.07 atm at a total pressure of 1 atm. Determine the degree of dissociation of SbCl5
SbCl3(g) + Cl2(g) is 1.07 atm at a total pressure of 1 atm. Determine the degree of dissociation of SbCl5
- 92 attempts
- 0 upvotes
- 2 comments
Dec 12CSIR NET & SET

