- Home/
- CDS & Defence/
- Article
Which of the following is an optically active compound – (a) n-Propanol (b) n-Butanol (c) 2-Chlorobutane (d) 4-Hydroxyheptane
By BYJU'S Exam Prep
Updated on: September 25th, 2023
An optically active compound is 2-Chlorobutane.
Super Impossibility of images –
In order for two images to be superimposed on one another, their corresponding sections must match. If one is positioned on top of the other. They are referred to as non-superimposable on their mirror image if they do not coincide.
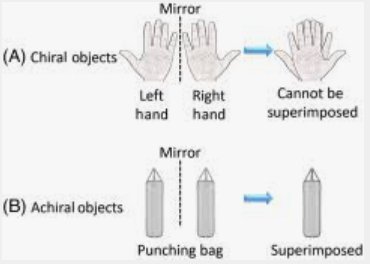
Table of content
Optical isomerism:
Optical isomers are two isomers that rotate plane polarised light in opposite directions. Optic isomerism is the term for the phenomenon.
Criteria to indicate optical isomerism:
Absence of the centre of symmetry: If a line were traced from a molecule’s centre to each atom’s corner, it shouldn’t run into any comparable atoms.
Absence of plane of symmetry – A molecule is divided by an imaginary or real, horizontal or vertical, plane as it is passed through it, preventing one half of the molecule from being the mirror image of the other. A molecule or entity that lacks a plane of symmetry and cannot be superimposed on the mirror image is considered to be dissymmetric or chiral if it has a chiral centre.
Let’s examine chiral centres in the following molecules.
Propanol:
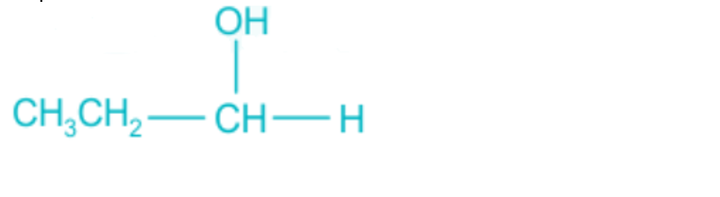
It is evident from the aforementioned structure that the centre of carbon is achiral and does not include four distinct groups. Propanol is not active optically as a result.
n-Butanol:
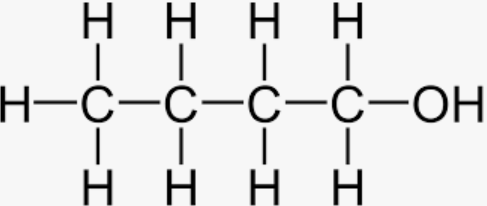
Since n-Butanol lacks a carbon centre with four distinct groups, it is not achiral. N-Butanol is not active optically as a result.
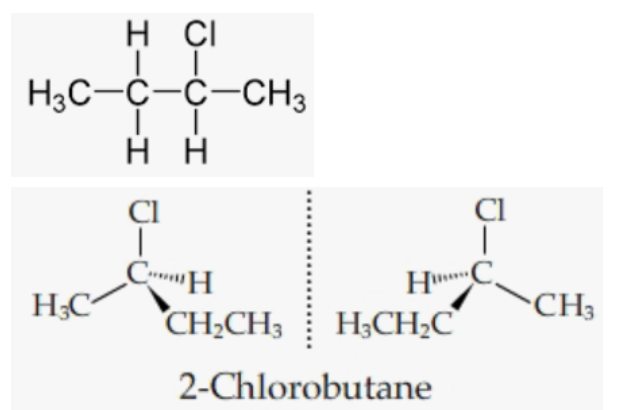
2-Chlorobutane:
Because 2-chlorobutane has a chiral carbon centre, it is active optically. Below are the mirror image:
4-Hydroxyheptane:
4-Hydroxyheptane has no chiral centre and is optically inactive. Therefore, an optically active compound is 2-Chlorobutane.
Summary:
Which of the following is an optically active compound – (a) n-Propanol (b) n-Butanol (c) 2-Chlorobutane (d) 4-Hydroxyheptane
2-Chlorobutane is an optically active compound.
