- Home/
- CDS & Defence/
- Article
Which of the following compounds will exhibit cis-trans (geometrical) isomerism? (a) 2-Butene (b) 2-Butyne (c) 2-Butanol (d) 1-Buetene
By BYJU'S Exam Prep
Updated on: September 25th, 2023
2-Butene will exhibit cis-trans (geometrical) isomerism. Isomers are substances with similar molecular formulas but various structural or stereochemical characteristics. According to their structural characteristics, organic molecules can be categorized in a wide range of ways.
Table of content
Cis and trans isomerism
- The term cis isomer refers to a geometrical isomer where two identical groups are located on the same side of the C = C.
- The trans isomer of a geometrical isomer is one in which the two identical groups are located on the other side of the C = C.
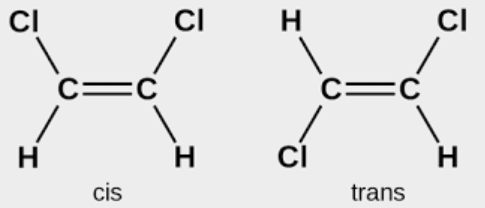
2 – Butene:
CH3-CH=CH-CH3 is the molecular formula It is of abC = Cab type and will exhibit cis-trans isomerism.

2 Butyne:
C4H6 is the molecular formula

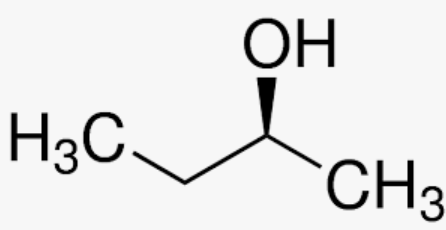
2 Butanol:
The molecule lacks a C=C bond. Therefore, does not meet the requirements to demonstrate geometric isomerism.
Butene:
The molecule does not have two distinct groups connected to it, but it does contain a C = C.
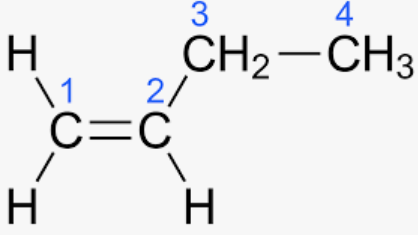
As a result, it fails to demonstrate geometric isomerism. Only 2 – Butene exhibits cis-trans isomerism as a result.
- The physical and chemical characteristics of the trans and cis isomers vary.
- In general, trans isomers have greater melting points than cis isomers.
- In the case of cis isomers, the dipole moment is higher.
Summary:
Which of the following compounds will exhibit cis-trans (geometrical) isomerism? (a) 2-Butene (b) 2-Butyne (c) 2-Butanol (d) 1-Buetene
Geometric isomerism of the cis-trans kind will be seen in 2-butene. Isomers are compounds with the same chemical structure but different stereochemical or structural properties. Organic molecules can be grouped in a variety of ways according on their structural features.
