Which of the Following Compounds on Oxidation gives Benzoic Acid?
By BYJU'S Exam Prep
Updated on: October 17th, 2023
(A) Chlorophenol
(B) Chlorotoluene
(C) Chlorobenzene
(D) Benzyl chloride
The compound Benzyl chloride forms benzoic acid on oxidation. The term “oxidation reaction” describes a reaction in which either oxygen is added, or hydrogen is removed. It is sometimes referred to as the loss of one or more electrons by atoms or ions. Example is: Mg + O2 = MgO2.
Table of content
Benzyl Chloride Forms Benzoic Acid on Oxidation
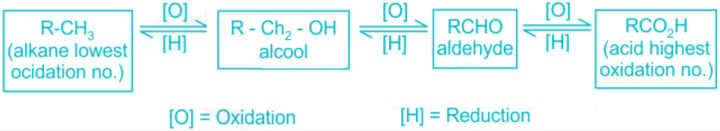
- Benzoic acid is created when benzyl chloride and alkyl benzenes are subjected to oxidation.
- It can be produced by a variety of oxidizing agents, including KMnO4, and K2Cr2O7, in the presence of acids such as H2SO4.
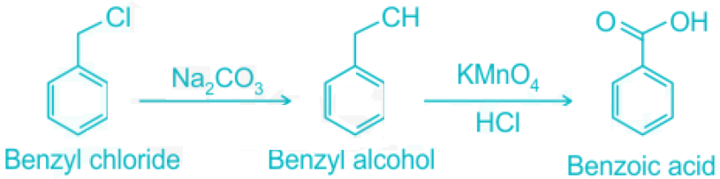
- Benzyl alcohol is created when sodium carbonate and benzyl chloride are mixed.
- Benzoic acid is created when benzyl alcohol is exposed to an oxidizing agent while also being in the presence of an acid.
The reactions are listed in the following order:
- Chlorobenzoic acid is produced by the side chain oxidation of chlorobenzene when it is subjected to oxidizing substances.

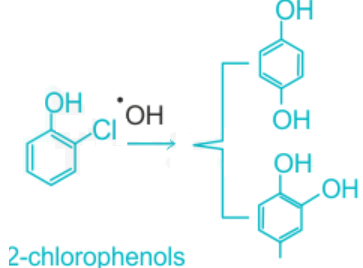
- Chlorophenols are converted into benzene diols through oxidation, which comes into equilibrium with quinones.
Benzyl chloride produces benzoic acid as a result of oxidation.
Summary:
Which of the following compounds on oxidation gives benzoic acid? (A) Chlorophenol (B) Chlorotoluene (C) Chlorobenzene (D) Benzyl chloride
Benzyl chloride produces benzoic acid when it is oxidized. The above-given chemical reactions will be helpful in understanding the process of Benzyl chloride oxidation and the formation of benzoic acid.


