What is the CFSE of the d7 electronic configuration in the presence of a strong field ligand?
By BYJU'S Exam Prep
Updated on: October 17th, 2023
(A) 1.2 Δ0
(B) 0.8 Δ0
(C) 0.4 Δ0
(D) 1.8 Δ0
In the presence of a strong field ligand, the CFSE of the d7 electronic configuration is 1.8 Δ0. As the ligands meet the metal ions in the complex’s formation, electrostatic repulsion happens among the ligands’ electrons and metal electrons. The electrostatic attraction also exists between the ligands electrons and the metal nucleus.
Table of content
CFSE of the d7 Electronic Configuration
The spherically symmetrical electrostatic field of the ligands alters the energies of the orbitals, resulting in a net increase in energy. The energy of the d orbitals has increased, but they are still degenerate or have similar energy.
If a complex will be a low spin or high spin, we can find it by the magnitude of CFSE. The energy of the ‘t2g’ orbitals is reduced by. 4Δ0 and the energy of ‘e’ is increased by .6Δ0 each.
- Strong field ligands typically result in more splitting and high CFSE.
- When this occurs, the electrons couple up in the lower orbitals set because the Pairing energy P is unable to defeat the CFSE.
- There are a fewlone electrons when the electrons pair up, making the complex a low spin.
- The d7 electronic configuration in strong field ligands presence is shown below:
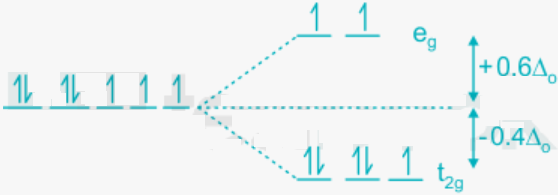
The CFSE = difference of energy between the two orbitals sets = lower set energy – higher set energy
= (.4 × 6) – (1 × .6) = 2.4 – .6 = 1.8Δ0
Therefore, the CFSE of the d7 electronic configuration in strong field ligand presence is 1.8Δ0.
Summary:
What is the CFSE of the d7 electronic configuration in the presence of a strong field ligand? (A) 1.2 Δ0 (B) 0.8 Δ0 (C) 0.4 Δ0 (D) 1.8 Δ0
The CFSE of the d7 electronic configuration in the presence of a strong field ligand is 1.8Δ0. The CFT treats ligands as the point charges and increases their degeneracy since d-orbitals are easily broken.


