- Home/
- CDS & Defence/
- Article
What are the limitations of J.J. Thomson’s model of the atom?
By BYJU'S Exam Prep
Updated on: September 25th, 2023
The limitations of J.J Thomson’s model of an atom is – it failed to explain the alpha particle scattering experiment results, no experimental pieces of evidence were not given by him and he failed to describe the atoms stability.
William Thomson put forth the Thomson atomic model in 1900. This model provided a theoretical explanation of the description of an atom’s interior structure. Sir Joseph Thomson, who had earlier made the discovery of the electron, backed it wholeheartedly.
J.J. Thomson found a negatively charged particle during a cathode ray tube experiment. In 1897, this experiment was conducted. A vacuum tube is a cathode ray tube. The electron was the name given to the harmful particle.
Postulates of J.J Thomson’s model of an atom:
- The electrons are housed inside a positively charged sphere that is part of an atom.
- Positive and negative charges are of equal magnitude. An atom is electrically neutral as a result of this.
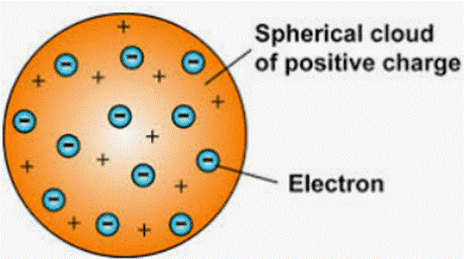
Table of content
Limitations of J.J Thomson’s model of an atom:
- The results of Rutherford’s alpha particle scattering experiment could not be explained by Thomson’s model.
- His concept has a number of significant flaws, one of which being the absence of any experimental data.
- He was unable to explain why atoms are stable.
Summary:-
What are the limitations of J.J. Thomson’s model of the atom?
The flaws in J.J. Thomson’s concept of an atom include that it was unable to explain the results of the alpha particle scattering experiment, he provided no experimental data, and he did not characterise the stability of the atom.
Related Questions:-
- A Person With a Myopic Eye Cannot See Objects Beyond 12 M Distinctly What Should Be the Type of Corrective Lens
- A Point Object is Placed at a Distance of 30 Cm From a Convex Mirror of Focal Length of 30 Cm the Image Will Form at
- Derive the Third Equation of Motion V2 Minus U2 Equal to 2as
- Find the Sum 1 Plus 2 Plus 3 Plus 4 Plus 5 Plus 100
- How Do You Balance the Equation Als Hcll Alcl3 S H2g
- How Do You Simplify 1 Plus Tan X 1 Minus Tan X
