VB Theories Full Form – Introduction To VB Theories, Postulates, Applications and Examples!
By BYJU'S Exam Prep
Updated on: September 13th, 2023

VB Theories Full Form: According to the valence bond theory, the electrons in a molecule occupy atomic orbitals rather than molecular orbitals. The electrons are localized in the bond region due to overlapping and the overlapping process of atomic orbitals results in the formation of a chemical bond. The metal bonding is necessary for covalent in origin, the metallic structure involves resonance of electron-pair bonds among each atom and its neighbors.
Scroll down the full article to acknowledge the basics of VB Theories Full Form, Postulates Of VB Theories, Applications and Examples on VB Theories.
Table of content
Introduction To VB Theories
The VB Theories have the following points-
- This theory explains chemical bonding in coordination compounds by using quantum mechanical concepts.
- VBT when applied to metal complexes represents an extension of its applications to polyatomic molecules.
- While applying VBT to coordination complexes, Pauling accepted Sidgwick’s idea that the formation of the complex is a result of coordinate bond formation between metal ions and ligands.
- It was given by Pauling in 1930.
- It deals with the covalent bonding between the donor atom of the ligand and the vacant hybrid orbital of the central metal ion.
Postulates
This theory can be understood under the following 3 postulates:
(I) Vacant atomic orbitals equal to their coordination number are made available by the central metal atom/ion.
Such vacant atomic orbitals hybridize to form a new set of equivalent bonding orbitals having definite directional character.
The most common hybridizations obtained are-
|
Coordination Number |
Hybridization |
Geometry |
Ions forming complexes |
|
6 |
d2sp3 |
Octahedral |
Sc(III), Ti(IV), V(III), Cr(III), Fe(II), Co(II), Co(III), Mn(II), Pt(IV) |
|
4 |
dsp2 |
Square planar |
Ni(II), Pd(II), Pt(II), Cu(II) |
|
4 |
sp3 |
Tetrahedral |
Zn(II), Cu(I), non-transition metal ions |
|
2 |
sp |
Linear |
Ag(I), Au(I) |
(II) The vacant hybrid orbitals of the metal overlap with filled orbitals of the donor group (ligands) to form a σ-bond.
This σ-bond is known as a Coordinate bond. It possesses a considerable amount of polarity because of the mode of its formation.
(III) Along with σ-bond, there is the possibility of the formation of π-bond.
π-bond is formed when the filled d-orbital of the metal overlaps with vacant orbitals on the ligand atom.
This π-bond helps to remove the excessive negative charge which would otherwise accumulate on the central metal atom. Here, electroneutrality is attained and the metal-ligand bond is strengthened.
Example: To explain the stability of [Co(H2O)6]+2
Pauling suggested that a (–4) electron charge is accumulated over the Cobalt atom because σ-bonding is redistributed among metal and ligands so that no atom in a complex bears a resultant charge greater than (–1) electron.
This unfavorable situation does not arise due to
(i) back bonding and
(ii) electroneutrality principle
Applications
It explains the following properties of the complex-
(a) Stereochemistry
(b) Geometry
(c) Magnetic properties
(d) Type of complex – inner orbital or outer orbital complex
(e) Hybridization and shape
(f) Nature of complex – high spin or low spin
(g) Effective Atomic Number (EAN)
Examples
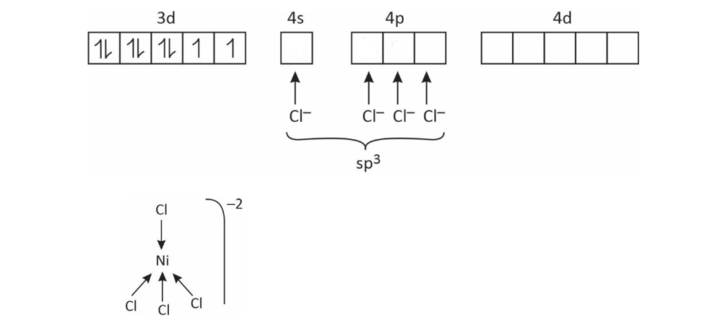
(1) [NiCl4]–2
Coordination number = 4
So, either sp3 or dsp2
x + (–4) = – 2
x = + 2
∴ Ni has +2 charge in this complex
Ni+2 = 3d8 4s°
EAN = 34
Tetrahedral, sp3
Paramagnetic
Outer orbital complex
High spin complex
(2) [Fe(CN)6]–4
Coordination number = 6
x + (–6) = –4
x + 2
∴ Fe has +2 charge in this complex
Fe+2 = 3d6 4s°
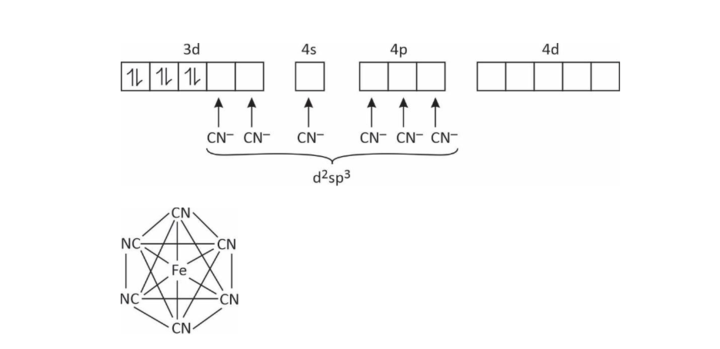
EAN = (26 – 2) + 2 × 6 = 24 + 12 = 36
Octahedral
n = 0; ∴ diamagnetic
μ = 0 BM
low spin complex
inner orbital complex
Limitations of VB Theory Full Form
VBT does not explain the following
(1) Color of ions in solution.
(2) Distorted structure of complexes.
(3) Basic criterion of formation of the inner orbital complexes.
(Spin paired or low spin) and outer orbital (spin-free or high spin) complexes.
(4) Quantitative value of the magnetic moment.
(5) Why does magnetic moment vary with temperature.
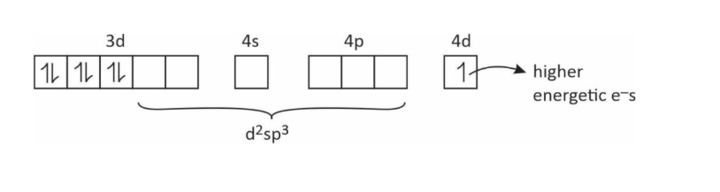
(6) How is it possible that one e– moves from (n – 1)d to nd orbital.
(7) Reducing the nature of some complexes.
[Co(NH3)6]+2 → [Co(NH3)6]+3 + e–
↓
Good reducing agent
More Full-Form Articles –
| PCR Full-Form | GPCR Full-Form |
| GISH Full-Form | ELISA Full-Form |


