The dihedral angle of the least stable conformer of ethane is : (a). 0° (b). 120° (c). 180° (d). 60°
By BYJU'S Exam Prep
Updated on: September 13th, 2023
We know that
Ethane is an organic Chemical Compound that has a colorless and odor gas at a standard temperature. The molecule consists of seven sigma bonds and there will be a change in the shape of the molecule when there is a rotation of about six carbon-hydrogen bonds.
There are two conformers in ethane i.e. eclipsed and staggered form.
Table of content
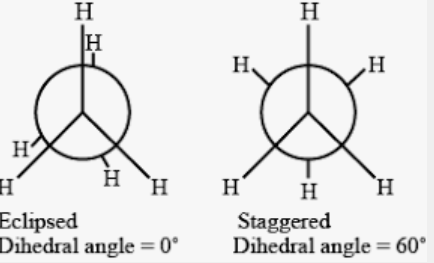
The eclipsed conformer is less stable whereas the staggered conformer is highly stable. The dihedral angle of the eclipsed conformer is 0° and the staggered conformer is 60°.
Therefore, the dihedral angle of the least stable conformer of ethane is 0°.
Summary:
The dihedral angle of the least stable conformer of ethane is : (a). 0° (b). 120° (c). 180° (d). 60°
The dihedral angle of the least stable conformer of ethane is 0°.
Related Questions:-


