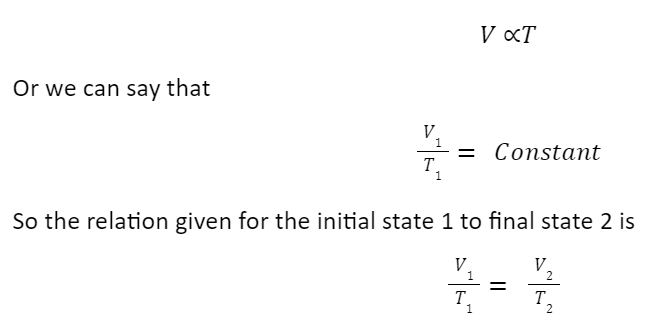- Home/
- GATE MECHANICAL/
- Article
Properties of Gases
By BYJU'S Exam Prep
Updated on: September 25th, 2023

Before entering into the properties of gases let us understand the different states of matter. There are three states of matter, and on the basis of their properties, shapes, and the effect of temperature and pressure they are classified as Solid, Liquid, and Gas. Solids have definite shape and volume, if we talk about liquids they have definite volume but do not have a definite shape they acquire the same shape as the container.
Table of content
Ideal Gas
The gas which follows all the laws of gases is known as an ideal gas, all gases at high temperatures are treated as an ideal gas.
Ideal Gas Equation
The relationship between the absolute temperature, pressure, volume, number of moles, and the characteristic gas constant is known as the Ideal gas equation. It is derived from the laws of gases and is given by
PV = n RT
R is the characteristic gas constant (R = 8.314 J/mol-K).
To understand the properties of gases, four variables are required, i.e., pressure, temperature, volume, and the number of moles. Various scientists give relationships between these variables called laws of gases, and based on these laws, we define the Ideal gas equation. Let us understand the various properties of gases.
Properties of Gases
As we know there is no definite shape or volume maintained by the gases, they acquire the same shape or volume as vacant space. The intermolecular forces between the molecules of the gases are very low, so molecules are free to move in vacant spaces and attain kinetic energy. Due to this weak bonding between the molecules, gases exerts some properties, and these are defined as the properties of gases.
Understanding the behavior of matter at its most basic level, individual particles, acting independently and almost entirely free of interactions and interferences with one another, is possible through studying the properties of gases.
What are Different Properties of Gases?
The most amazing thing about gases is that they all respond to temperature and pressure changes in the same way, expanding or shrinking in predictable quantities. Each gas has its different properties, and these properties of gases are known as
- Compressibility
- Diffusibility
- Expansivity
- Exertion of Pressure
- Low density
Compressibility
As the molecules of the gases move freely in a vacant space there will be large intermolecular spaces between the particles, on compressing the gases these intermolecular spaces reduce which results in the reduction of the volume. Because of these intermolecular spaces, gases are highly compressible, and the property is known as compressibility.
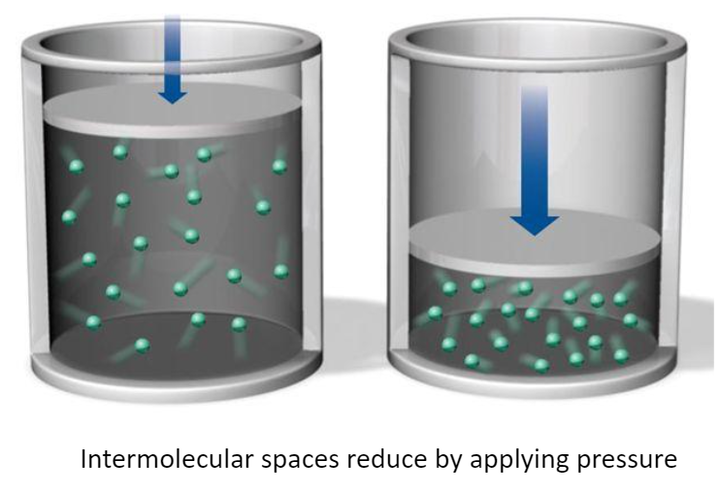
Expansivity
As we know, on compressing the gas, molecules come closer, and the property is known as compressibility. similarly, on removing pressure, the gas will expand, and volume will increase.
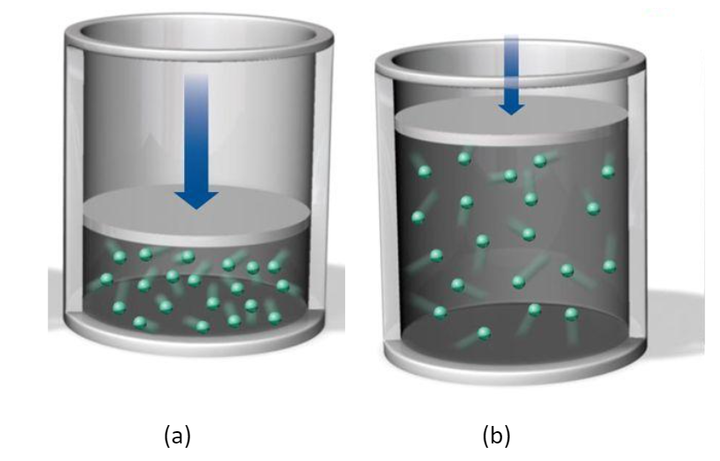
At position b if we increase the temperature of the gas by providing some external source the molecules gain energy and start moving faster and the intermolecular forces between the molecules also get reduced this lead to the further expansion of the gas and volume will increase.
Diffusibility
The gases can mix completely with the other gas homogeneously. If two gases A and B are separated by a thin wall in a container, on removing separation molecules of both the gases will pass through the intermolecular spaces without requiring any external work and get mixed homogeneously. This property of gases is known as Diffusibility.
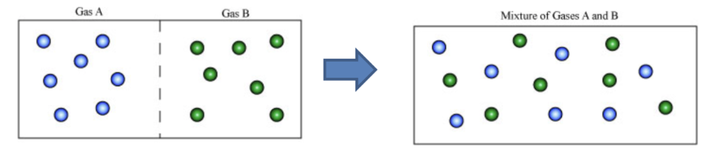
Low Density
Due to vast intermolecular spaces, the density of the gases is very low. For example, if we take V volume of water at temperature T and pressure P on transforming it into a gaseous form the volume will increase 16 to 17 times but the mass remains the same, due to this increased volume density of gases are low.
Exertion of Pressure
The molecules of the gases move freely and apply equal pressure in all directions by the bombardment of particles on the walls of the container or any closed object.
Why is it Important to Study the Properties of Gases?
The properties of gases are fundamental as it defines the behavior of gases under different conditions. Through these properties of gases, one can suggest which gas should be chosen for a particular condition. For example, we use gases in refrigerators, ACs, Power Plants, etc., based on the properties of the gases they are selected.
In contrast to liquids and solids, gases have lower densities, one of their standout characteristics. At 298 K and 1 atm pressure, a mole of liquid water has a volume of 18.8 cm3, whereas a mole of water vapor under the same conditions has a volume of 30200 cm3, which is more than 1000 times larger.
Gas Laws
The physical conditions of any gas are defined by its Absolute temperature ( T), pressure (P), volume (V), and the number of moles of the gas (n). To determine the physical conditions various relationships of these variables are given by different scientists. The relationship between these variables is known as gas laws.
- Boyle’s law
- Avogadro’s law
- Charles’s law
Boyle’s Law
It states that for a constant temperature, the volume of the gas is inversely proportional to its pressure.

Avogadro’s Law
It states that for a constant temperature and pressure, the volume of gas is directly proportional to its amount or number of moles (n).
V ∝ η
Charles’s Law
It states that for constant pressure, the volume of gas is directly proportional to its temperature.