Petrochemicals refers to all those compounds that can be derived from the petroleum refinery products
Typical feedstocks to petrochemical processes include
- C1 Compounds: Methane & Synthesis gas
- C2 Compounds: Ethylene and Acetylene
- C3 Compounds: Propylene
- C4 Compounds: Butanes and Butenes Aromatic Compounds: Benzene
It can be seen that petrochemicals are produced from simple compounds such as methane, ethylene and acetylene but not multi-component products such as naphtha, gas oil etc.
Classification: Petrochemicals can be broadly classified into three categories-
- Light Petrochemicals: These are mainly used as bottled fuel and raw materials for other organic chemicals. The lightest of these -- methane, ethane and ethylene -- are gaseous at room temperature.The next lightest fractions comprise petroleum ether and light naphtha with boiling points between 80 and 190 degrees Fahrenheit.
- Medium Petrochemicals: Hydrocarbons with 6 – 12 carbon atoms are called "gasoline", which are mainly used as automobile fuels. Octane, with eight carbons, is a particularly good automobile fuel, and is considered to be of high quality. Kerosene contains 12 to 15 carbons and is used in aviation fuels, and also as solvents for heating and lighting.
- Heavy Petrochemicals: These can be generally categorized as diesel oil, heating oil and lubricating oil for engines and machinery. They contain around 15 and 18 carbon atoms with boiling points between 570 and 750 degrees Fahrenheit. The heaviest fractions of all are called "bitumens" and are used to surface roads or for waterproofing. Bitumens can also be broken down into lighter hydrocarbons using a process called "cracking."
Manufacturing of Methanol from Synthesis Gas
- Synthesis gas is H2+CO
- When synthesis gas is subjected to high pressure and moderate temperature conditions, it converts to methanol.
- Followed by this, the methanol is separated using a series of phase separators and distillation columns.
- The process technology is relatively simple.
Reactions:
Desired: CO + 2H2→ CH3 OH
Side reactions:
CO + 3H2→ CH4 + H2 O 2CO + 2H2→ CH4 + CO2
All above reactions are exothermicUndesired reaction: zCO + aH2→ alchohols + hydrocarbons.
- Undesired reaction: zCO + aH2→ alchohols + hydrocarbons.
- Catalyst: Mixed catalyst made of oxides of Zn, Cr, Mn, Al.
Process Technology (Figure)
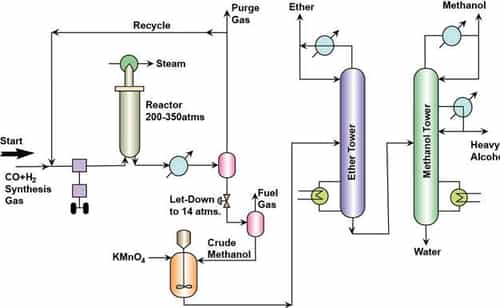
Figure 13.1 Flow sheet of manufacture of Methanol from Synthesis Gas
- H2 and CO adjusted to the molar ratio of 2.25.
- The mixture is compressed to 200 – 350 atms
- Recycle gas (Unreacted feed) is also mixed and sent to the compressor.
- Then eventually the mixture is fed to a reactor. Steam is circulated in the heating tubes to maintain a temperature of 300 – 375°C.
- After reaction, the exit gases are cooled.
- After cooling, phase separation is allowed. In this phase separation operation methanol and other high molecular weight compounds enter the liquid phase and unreacted feed is produced as the gas phase.
- The gas phase stream is purged to remove inert components and most of the gas stream is sent as a recycle to the reactor.
- The liquid stream is further depressurized to about 14 atms to enter a second phase separator that produces fuel gas as the gaseous product and the liquid stream bereft of the fuel gas components is rich of the methanol component.
- The liquid stream then enters a mixer fed with KMNO4 so as to remove traces of impurities such as ketones, aldehydes etc.
- Eventually, the liquid stream enters a distillation column that separates dimethyl ether as a top product.
- The bottom product from the first distillation column enters a fractionator that produces methanol, other high molecular weight alcohols and water as three different products.
Formaldehyde
Formaldehyde is produced from methanol
Reactions
- Oxidation: CH3OH + 0.5 O2 → HCHO + H2O
- Pyrolysis: CH3OH → HCHO + H2
- Undesired reaction: CH3OH + 1.5 O2 → 2H2O + CO2
- In the above reactions, the first and third are exothermic reactions but the second reaction is endothermic. The reactions are carried out in vapour phase. Catalyst: Silver or zinc oxide catalysts on wire gauge are used.
- Operating temperature and pressure: Near about atmospheric pressure and 500 – 600°C
Chloromethanes
- Chloromethanes namely methyl chloride (CH3Cl), methylene chloride (CH3Cl2), Chloroform (CHCl3) and Carbon Tetrachloride (CCl4) are produced by direct chlorination of Cl2 in a gas phase reaction without any catalyst.
Reactions
- CH4 + Cl2 → CH3Cl + HCl
- CH3Cl + Cl2 → CH2Cl2 + HCl
- CH2Cl2 + Cl2 → CHCl3 + HCl
- CHCl3 + H2 → CCl4 + HCl
- The reactions are very exothermic.
- The feed molar ratio affects the product distribution. When CH4/Cl2 is about 1.8, then more CH3Cl is produced. On the other hand, when CH4 is chosen as a limiting reactant, more of CCl4 is produced. Therefore, depending upon the product demand, the feed ratio is adjusted.
Isopropanol
Isopropanol is manufactured from hydration of propylene.
- Acetone is produced using the dehydrogenation route of isopropanol.
Reaction
- Sulfation: CH3CHCH2+ H2SO4→(CH3 )2 CH(OSO3H) (Isopropyl acid sulphate).
- Hydrolysis: Isopropyl sulphate +H2O→ Isopropanol + Sulfuric acid.
- Thus sulphuric acid is regenerated in the process.
Side reaction
- Diisopropyl sulphate + H2O→ Diisopropyl ether + Sulfuric acid.
- Therefore, the primary reaction is a gas liquid reaction in which propylene is absorbed into a tray tower fed with sulphuric acid.
- Operating conditions: Room temperature but 20 – 25 atms pressure.
- Reaction is highly exothermic.
Uses of Isopropanol
- There may be many uses of iso-propanol, industrial as well as common uses. It finds use in pharmaceutical applications because of the low toxicity of any residues. Isopropanol is also used as a chemical intermediate in some industrial processes. It is also used as a gasoline additive.
Acetone
- Acetone is manufactured from isopropanol
Reactions
- Dehydrogenation of Isopropanol
- Isopropanol → Acetone + H2
- Reaction pressure: 3 – 4 atms
- Reaction temperature: 400 – 500oC
- Copper catalyst on porous carrier is used
- Vapor phase reaction
Uses of Acetone
- Acetone is used as a polar, aprotic solvent in a variety of organic reactions. One important property for which it is used as laboratory solvent is because does not form an azeotrope with water.
- Acetone is also used in various medical and cosmetic applications. It also forms an important component in food additives and food packaging.
Phenol from benzene
Phenol can be manufactured from Benzene using several ways
- Benzene hydro chlorination to form Benzyl chloride followed by hydrolysis of benzyl chloride to form phenol.
- Benzene chlorination to form benzyl chloride which is transformed to sodium benzoate and eventually to phenol using NaOH and HCl
- Benzene sulfonate process: In this process, benzene is convered to benzene sulfonate using sulphuric acid and eventually through neutralization, fusion and acidification, the benzene sulfonate is gradually transformed to phenol.
Phenol using Hydro chlorination route
Reactions
- First reaction
- Benzene + HCl + Oxygen → Benzyl chloride + Water
- Catalyst: FeCl3 + CuCl2
- Operating conditions: 240°C and atmospheric pressure
- Second reaction
- Benzyl chloride + water → Phenol + HCl
- Catalyst: SiO2
- Here, HCl is regenerated and will be recycled.
- Operating conditions: 350°C and atmospheric pressure
Phenol from Chlorobenzene route
There are three reactions to convert benzene to phenol using chlorination route
- Chlorination
- Benzene + Cl2 → monochloro benzene
- Operating Temperature: 85°C
- Catalyst: Fe or FeCl3 catalyst
- Causticization
- Benzyl chloride + NaOH → sodium benzoate
- NaOH is in aqueous media
- Operating conditions: 425°C and 350 atms
Styrene and Phthalic Anhydride
Styrene is produced from benzene via the ethyl benzene route followed by dehydrogenation.
The phthalic anhydride is produced from Naphthalene and o-Xylene.
- Styrene
Reactions :
- Alkylation of Benzene
- Benzene + ethylene → Ethyl benzene
- Catalyst: AlCl3
- C2 H5Cl provides hydrogen and chlorine free radicals.
- Operating conditions: 95°C and 1 atm pressure.
- Reaction is exothermic.
- Dehydrogenation of ethylbenzene
- Ethylbenzene à Styrene + Hydrogen.
- Reaction is endothermic.
- Catalyst: SnO or FeO.
- Operating conditions: 800°C.
Uses of styrene
- Styrene is mainly used for making plastic toys and model kits. Moreover, housing for machines as well as refrigerator doors and air conditioner cases are made of styrene
Phthalic Anhydride
Uses of Phthalic anhydride :
- Phthalic anhydride is used as a versatile intermediate in organic chemical reactions, mainly because it is bifunctional and is cheaply available.
- It may also be used in the manufacture of phthalate plasticizers like DOP, DEP etc.
Maleic Anhydride and DDT
- Maleic anhydride is manufacture from benzene by butane oxidation.
- DDT is a pesticide and is manufactured from benzene, chlorine and ethanol using sulphuric acid as a catalyst.
Maleic anhydride & Fumaric acid
Reactions
- Benzene + O2 (Air) → Maleic anhydride + H2O + CO
- The reaction is exothermic.
- Operating temperature is 400 – 500°C.
- Catalyst is V2O5
- For fumaric acid, the reaction is Maleic acid → Fumaric acid.
- Fumaric acid is an isomer of Maleic acid.
- HCl is used as a catalyst for the isomerization reactor at normal pressure and temperature.
Uses
- Maleic anhydride can be used as a highly reactive and versatile raw material.
- It can be used in the manufacture of alkyd resins, which in turn are used for making paints and coatings.
- It can also be used in making agricultural chemicals like herbicides, pesticides and plant growth regulators.
PETROLEUM REFINING
Crude oil
Crude oil is a multicomponent mixture consisting of more than 108 compounds. Petroleum refining refers to the separation as well as reactive processes to yield various valuable products. Therefore, a key issue in the petroleum refining is to deal with multicomponent feed streams and multicomponent product streams.
Feed and Product characterization
The physical characterization of the crude oil in terms of viscosity, density, boiling point curves is equally important. These properties are also indicative of the quality of the product as well as the feed. Therefore, in petroleum processing, obtaining any intermediate or a product stream with a defined characterization of several properties indicates whether it is diesel or petrol or any other product.
Important characterization properties are
1. API gravity
API gravity of petroleum fractions is a measure of density of the stream. Usually measured at 60oF, the API gravity is expressed as
o API = [141.5/specific gravity ] – 131.5
Where specific gravity is measured at 60o F.
2. Watson characterization factor
The Watson characterization factor (K) is usually expressed as

Where TB is the average boiling point in degrees R taken from five temperatures corresponding to 10, 30, 50,70 and 90 volume % vaporized.
Typically Watson characterization factor varies between 10.5 and 13 for various crude streams. A highly paraffinic crude typically possesses a K factor of 13. On the other hand, a highly naphthenic crude possesses a K factor of 10.5. Therefore, Watson characterization factor can be used to judge upon the quality of the crude oil in terms of the dominance of the paraffinic or naphthenic components.
3. Viscosity
Viscosity is a measure of the flow properties of the refinery stream. Typically in the refining industry, viscosity is measured in terms of centistokes (termed as cst) or saybolt seconds or redwood seconds. Usually, the viscosity measurements are carried out at 100oF and 210oF. Viscosity is a very important property for the heavy products obtained from the crude oil. The viscosity acts as an important characterization property in the blending units associated to heavy products such as bunker fuel. Typically, viscosity of these products is specified to be within a specified range and this is achieved by adjusting the viscosities of the streams entering the blending unit.
4. Flash and fire point
Flash and fire point are important properties that are relevant to the safety and transmission of refinery products. Flash point is the temperature above which the product flashes forming a mixture capable of inducing ignition with air. Fire point is the temperature well above the flash point where the product could catch fire. These two important properties are always taken care in the day to day operation of a refinery.
5. Pour point
When a petroleum product is cooled, first a cloudy appearance of the product occurs at a certain temperature. This temperature is termed as the cloud point. Upon further cooling, the product will cease to flow at a temperature. This temperature is termed as the pour point. Both pour and cloud points are important properties of the product streams as far as heavier products are concerned. For heavier products, they are specified in a desired range and this is achieved by blending appropriate amounts of lighter intermediate products.
6. Octane number
The knocking tendency of the gasoline is defined in terms of the maximum compression ratio of the engine at which the knock occurs. Therefore, high-quality gasoline will tend to knock at higher compression ratios and vice versa. However, for comparative purpose, still one needs to have a pure component whose compression ratio is known for knocking. Iso-octane is eventually considered as the barometer for octane number comparison. While iso-octane was given an octane number of 100, n-heptane is given a scale of 0. Therefore, the octane number of a fuel is equivalent to a mixture of an iso-octane and n-heptane that provides the same compression ratio in a fuel engine. Thus an octane number of 80 indicates that the fuel is equivalent to the performance characteristics in a fuel engine fed with 80 vol % of isooctane and 20 % of n-heptane.
7. Crude composition
Fundamentally, crude oil consists of 84 – 87 wt % carbon, 11 – 14 % hydrogen, 0 – 3 wt % sulphur, 0 – 2 wt % oxygen, 0 – 0.6 wt % nitrogen and metals ranging from 0 – 100 ppm. Understanding thoroughly the fundamentals of crude chemistry is very important in various refining processes.
Based on chemical analysis and existence of various functional groups, refinery crude can be broadly categorized into various categories summarized as
Paraffins: Paraffins refer to alkanes such as methane, ethane, propane, n and iso butane, n and iso pentane. These compounds are primarily obtained as a gas fraction from the crude distillation unit.

Methane(CH4) Ethane(C2H6) Propene(C3H8) Normal Butane(nC4H10)

Normal Pentene (C5H12)
Olefins: Alkenes such as ethylene, propylene and butylenes are highly chemically reactive. They are not found in mentionable quantities in crude oil but are encountered in some refinery processes such as alkylation.

Ethylene(C2H4) Propylene(C3H6) Butylene(C4H8)
Naphthenes: Naphthenes or cycloalkanes such as cyclopropane, methyl cyclohexane are also present in the crude oil. These compounds are not aromatic and hence do not contribute much to the octane number. Therefore, in the reforming reaction, these compounds are targeted to generate aromatics which have higher octane numbers than the naphthenes.

Cyclopropane(C3H6) Cyclobutane(C4H8) Cyclopentane(C5H10)

Cyclohexane(C6H12) Methyl Cyclohexane(C7H14)
Aromatics: Aromatics such as benzene, toluene o/m/p-xylene are also available in the crude oil. These contribute towards higher octane number products and the target is to maximize their quantity in a refinery process.

Benzene(C6H6) Tolune(C7H8) Para-X ylene(C8H1

Ortho-Xylene(C8H10) Meta-X ylene(C8H10)
Napthalenes: Polynuclear aromatics such as naphthalenes consist of two or three or more aromatic rings. Their molecular weight is usually between 150 – 500.

Organic sulphur compounds: Not all compounds in the crude are hydrocarbons consisting of hydrogen and carbon only. Organic sulphur compounds such as thiophene, pyridine also exist in the crude oil. The basic difficulty of these organic sulphur compounds is the additional hydrogen requirements in the hydrotreaters to meet the euro III standards. Therefore, the operating conditions of the hydrotreaters is significantly intense when compared to those that do not target the reduction in the concentration of these organic sulphur compounds. Therefore, ever growing environmental legislations indicate technology and process development/improvement on the processing of organic sulphur compounds.
Oxygen containing compounds: These compounds do not exist 2 % by weight in the crude oil. Typical examples are acetic and benzoic acids. These compounds cause corrosion and therefore needs to be effectively handled.
Resins: Resins are polynuclear aromatic structures supported with side chains of paraffins and small ring aromatics. Their molecular weights vary between 500 – 1500. These compounds also contain sulphur, nitrogen, oxygen, vanadium and nickel.
Asphaltenes: Asphaltenes are polynuclear aromatic structures consisting of 20 or more aromatic rings along with paraffinic and naphthenic chains. A crude with high quantities of resins and asphaltenes (heavy crude) is usually targeted for coke production.
UNITS IN REFINERY
The various units presented in the refinery process diagram are categorized as
- Crude distillation unit (CDU)
- Vacuum distillation unit (VDU)
- Thermal cracker
- Hydrotreaters
- Fluidized catalytic cracker
- Separators
- Naphtha splitter
- Reformer
- Alkylation and isomerization
- Gas treating
- Blending pools
- Stream splitters
Crude distillation unit (CDU)
- The first essential task for the crude oil consisting of more than 108 compounds is to separate its major components based on boiling point differences. This principle is exploited in the crude distillation unit which involves energy intensive operation. Since crude distillation involves the processing of the entire feed, it remains as the most significant operation in a refinery.

Figure. Process flowsheet-a conceptual diagram of the crude distillation unit (CDU) with HEN(heat exchanger network)
The conceptual process flowsheet for the petroleum refinery is shown in the Figure .It consists of the following important sub-processes:
- Crude desalte
- Furnace
- Pre-flash column
- Crude distillation column supplemented with side columns.
- These columns produce the desired products
- Pump around heat exchanger units
- Heat exchanger network that facilitates energy recovery from hot product and reflux streams to heat the crude oil.
CRACKING
- A critical observation of the overall refinery process block diagram indicates that the straight run gasoline (this is the gasoline obtained from the CDU) does not have good octane number (40 – 60) and needs to be upgraded to obtain the desired octane number (85 – 95). Typically, cracking, reforming and isomerisation are regarded as the three most important of processes that contribute towards upgradation
|
|
|
|
| the | octane | number. |
In this lecture, we present an overview of the cracking operation in the refinery. |
|
| |||||
Typically cracking involves the thermal or catalytic decomposition of petroleum fractions having huge quantities of higher molecular weight compounds. Since heat is required, typically cracking reactions are carried out in furnaces that are supplied with either fuel oil or fuel gas or natural gas or electricity as heat source.
• Cracking involves the decomposition of heavier hydrocarbon feedstocks to lighter hydrocarbon feed stocks.
• Cracking can be carried out to any hydrocarbon feedstock but it is usually applied for vacuum gas oil
(VGO)
• Cracking can be with or without a catalyst.
• When cracking is carried out without a catalyst higher operating temperatures and pressures are required. This is called as thermal cracking. This was the principle of the old generation refineries.
• Now a days, cracking is usually carried out using a catalyst. The catalyst enabled the reduction in operating pressure and temperature drastically.
Cracking chemistry
Cracking is an endothermic reaction in which:
• Long chain paraffins converted to olefins and olefins
• Straight chain paraffins converted to branched paraffins
• Alkylated aromatics converted to aromatics and paraffins
• Ring compounds converted to alkylated aromatics
• Dehydrogenation of naphthenes to aromatics and hydrogen
Undesired reaction: Coke formation due to excess cracking
Therefore, in principle cracking generates lighter hydrocarbons constituting paraffins, olefins and aromatics. In other words, high boiling low octane number feed stocks are converted to low boiling high octane number products.
Operating conditions
• These very much depend upon the feed stock and type of cracking (thermal /catalytic ) used.
• Cracking is a gas phase reaction. Therefore, entire feedstock needs to be vaporized.
• It was observed that short reaction times (to the order of 1 – 3 seconds only) provide good quality product and less coke formation.
• For vacuum gas oil, thermal cracking requires operations at 600°C and 20 atms gauge pressure.
• For vacuum gas oil, catalytic cracking is usually carried out at 480°C and 0.7 – 1 atms gauge pressure.
Catalyst
• Acid treated silica-alumina was used as catalyst.
• 20 – 80 mesh size catalysts used for FCCR and 3 – 4 mm pellets used for MBRs
• During operation, poisoning occurs with Fe, Ni, Vd and Cu
Process technology
The process technology consists of two flowsheets namely the cracking coupled with main distillation column and stabilization of naphtha.
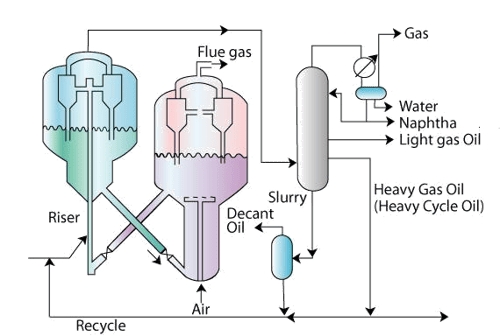
Figure 6.1 Flow sheet of Catalytic Cracking process
• Feed enters the cracking reactor.
• Old generation refineries used moving bed reactors
• Now a days, fluidized catalytic cracking (FCC) reactors are used.
• The cracked product from the reactor enters a main distillation column that produces unstabilized naphtha, light gas oil, heavy gas oil, slurry and gas.
Fluidized catalytic cracking reactor (FCCR) : (Figure 6.2)
• The basic principle of the FCCR is to enable the fluidization of catalyst particles in the feed stream at desired pressure and temperature.
• Another issue for the FCCR is also to regenerate the catalyst by burning off the coke in air.
• Therefore, the reactor unit should have basically two units namely a reactor (FCCR) and a catalyst regenerator (CR).
• The FCCR consists essentially of two important components in a sophisticated arrangement. These are the riser and the cyclone unit assembled in a reactor vessel.
Figure6.2 Fluidized Catalytic Cracking Reactors
• Riser: In the riser (a long tube), the feed is allowed to get in contact with the hot catalyst. The hot catalyst is enabled to rise through lift media in the riser. The lift media is usually steam or light hydrocarbon gas.
• The riser contact time is about 250 milliseconds.
• The riser is eventually connected to cyclone units.
• The cyclone units receive the catalyst and finished product. The catalyst that enters the cyclone unit is fully coked and needs to be sent to a regenerator to regain its lost activity.
• After cyclone operation (which separates the hydrocarbon vapors and catalyst as a solid fluid operation), the catalyst falls down to the vessel that houses the riser and cyclone units.
• The catalyst in the vessel is subjected to stream stripping in which direct contact with steam is allowed to remove hydrocarbons from the catalyst surface.
Catalyst regenerator (CR)
• The spent catalyst which is relatively cold enters the regenerator unit.
• Here air enters the vessel through a sparger set up.
• The catalyst is subsequently burnt in the air. This enables both heating the catalyst (which is required to carry out the endothermic reaction) and removing the coke so as to regain the activity of the coke.
• The catalyst + air after this operation will enter the cyclone separator unit. Unlike the FCCR, the CR does not have a riser. Therefore, air enters a dense phase of catalyst and also enables the movement of the catalyst to a dilute phase of catalyst + air
• The cyclone separators separate the flue gas and catalyst as a solid fluid operation.
• The activity regained catalyst is sent to the riser through a pipe.
• During this entire operation, the catalyst temperature is increased to 620 – 750°C
• The flue gas is obtained at 600 - 760°C and is sent for heat recovery unit to generate steam.
3. Reforming
The Reforming involves enhancing the octane number of the product
• Heavy naphthas are used are typical feed stocks
• The reaction is carried out on a catalyst
• Reforming reaction produces hydrogen as a by product which is used elsewhere in the refinery
• Usually Platinum supported on porous alumina is used as a catalyst
• Catalyst activity enhanced using chloride
Reforming Chemistry
• Paraffin isomerisation takes place
• Naphthene isomerisation also takes place to produce cycloalkanes
• Cyclo alkanes undergo dehydrogenation to generate aromatics




Comments
write a comment