- Home/
- CDS & Defence/
- Article
Out of the following, the most stable resonating structure is?
By BYJU'S Exam Prep
Updated on: September 25th, 2023

Out of the following, the most stable resonating structure is ![]()
Resonance
- We can claim that this is a hypothetical state in a chemical entity where two or more Lewis dot formations, each with a different electronic arrangement, can represent different electronic structures.
- Resonating structures are the names given to each structure.
- A hybrid form called resonance shows the characteristics of all the resonating structures.
Criteria to indicate resonance
- Compounds with the potential for electron delocalization serve as examples.
- In conjugated systems with alternating single or double bonds, delocalization is observed.
- Delocalisation can happen through :
- π-π orbital overlap
- π-p orbital overlap
- π-σ orbital overlap
- Resonance is impossible in structures in which the electrons are confined.
- The plane on which all the atoms undergo resonance should be placed.
Characteristics of the resonance Hybrid
- It is claimed that a resonance hybrid is highly stable when compared to any individual contributing structures.
- The stability of a molecule increases with more contributing structures.
- The more bonds a resonance structure has, the more it will contribute to the hybrid stability; not all resonance structures are created equal.
- The bond lengths in a resonance hybrid are never the same as those of the contributing structure.
- The structures that contribute the most are those in which the positive charge is located on the most electropositive structure and the negative charge is located on the most electronegative atoms.
- The formations with opposite charges on nearby atoms are more beneficial.
- The resonance hybrid is not significantly affected by the contributing structures that involve charge separation.
Structures 1 and 3 will be more stable and useful since they have four covalent bonds while structures 2 and 4 only have three.
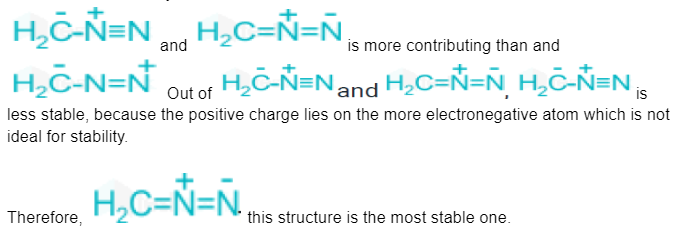
Summary:
Out of the following, the most stable resonating structure is?
the most stable resonating structure is 
