What is the Full Form of IUPAC in Chemistry? IUPAC Stands for?
By BYJU'S Exam Prep
Updated on: September 13th, 2023

IUPAC Full Form: International Union Of Pure and Applied Chemistry (IUPAC) was established in the Year 1919 and got recognized as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. The member of IUPAC is from the National Adhering Organizations, national academies of sciences can be national chemistry societies, or other bodies that represent the chemists. IUPAC is very well known for its contribution to working on the standardizing nomenclature in chemistry. However, IUPAC has its publications in many other scientific fields which include chemistry, biology, and physics.
Scroll Down the full article to acknowledge the Definition, and Naming of Alkanes in the IUPAC Full Form.
Introduction Of The IUPAC Full Form
In Chemical nomenclature, the IUPAC nomenclature of organic chemistry is used to name organic compounds. It is generally a set of logical rules that are used by organic chemists. By knowing these rules, one is easily able to write a unique name for every distinct compound.
IUPAC Full Form: The naming of Alkanes
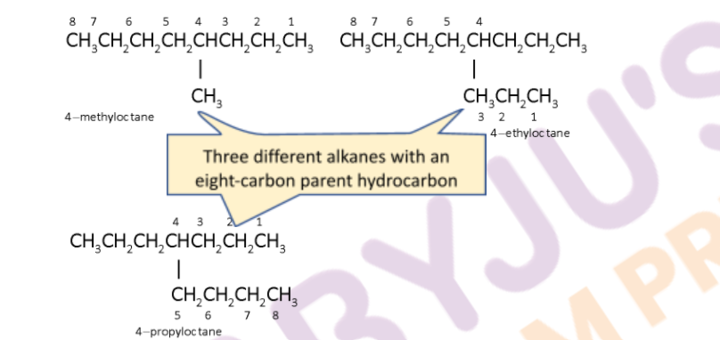
- First, determine the longest continuous carbon chain. This chain is known as the parent hydrocarbon. The number of carbon atoms present in the longest chain becomes the alkane’s suffix. For example, a chain having eight C atoms will be known as an octane. It is not necessary that the longest chain will be in a straight line. An example is:
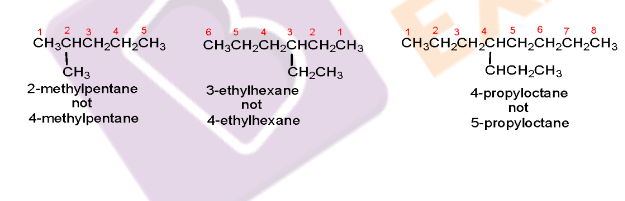
2. The name of an alkyl substituent will be placed in front of the parent hydrocarbon name with a number that indicates the carbon atom to which it is attached. The carbons in the parent chain are numbered in such a way that the substituent gets the lowest possible number. An example is:
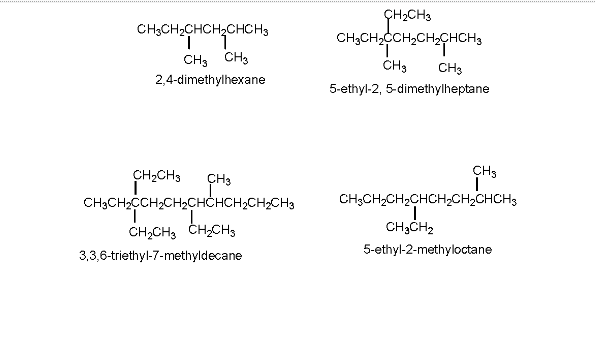
3. In the presence of more than one substituent, these will be listed in alphabetical order with each substituent preceded by the appropriate number. If the same substituents are present (two or more), the prefixes “di,” “tri,” and “tetra” are used to indicate the identical substituents the compound contains. The numbers indicate the locations of the identical substituents listed together, separated by commas. There will be no spaces on either side of a comma.
4. While numbering, if in either direction leads to the same lowest number for one of the substituents, the chain will be numbered in such a way that gives the lowest possible number to the remaining substituents.
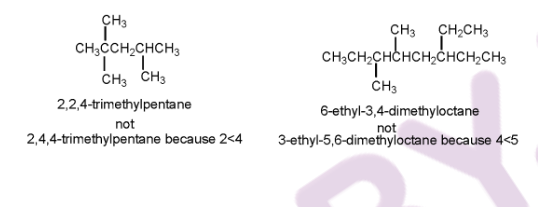
5. If in both directions, the same substituent numbers are obtained, then the first group listed receives the lower number.
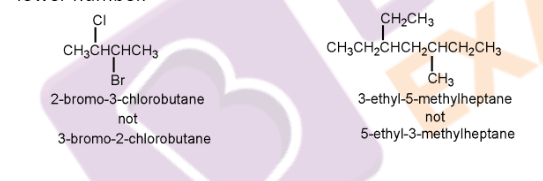
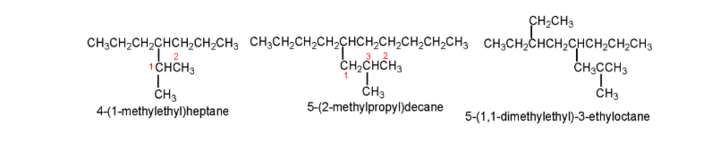
6. To name the branched substituents, first, do the numbering of alkyl substituents present at the carbon attached to the parent hydrocarbon. For these compounds, parenthesis is used. The number present in this parenthesis indicates the position of the substituent, while the number present outside it indicates the position of the parent hydrocarbon.
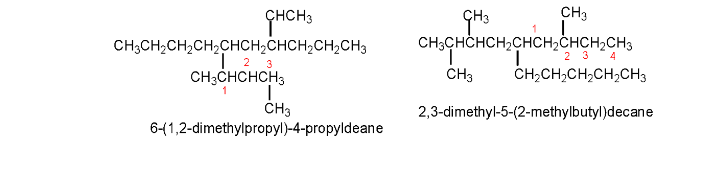
7. If a compound contains two or more chains of the same length, the parent hydrocarbon will be the chain having the greatest number of substituents.
IUPAC Full-Form – Click Here to Download PDF
Check Out:
- Previous Year’s Papers for CSIR-NET Exam: Attempt Here
- Study Notes for CSIR-NET Chemical Science – Download PDF Here
- Study Notes for CSIR-NET Life Science – Download PDF Here
More Full-Form Articles –
| PCR Full-Form | GPCR Full-Form |
| GISH Full-Form | ELISA Full-Form |
Stay Tuned for More Such Articles !!
BYJU’S Exam Prep App!
Download the BYJU’S Exam Prep App Now.
The Most Comprehensive Exam Prep App.
#DreamStriveSucceed
App Link:https://bit.ly/3sxBCsm


