GUS and GFP Full Form: Principle, Applications and Advantages
By BYJU'S Exam Prep
Updated on: September 13th, 2023

GUS and GFP Full Form: The GUS gene system is a reporter gene assay. The GUS gene was initially developed as a gene fusion marker in E. coli and in the nematode C.elegans, but now it is mostly used to monitor the chimeric gene expression in plants. The reporter gene is uid A from E. coli that encodes the enzyme b-glucuronidase (GUS), while the GFP is an autofluorescent protein that is used as a useful marker that helps to detect a target gene (encodes the protein of our interest).
We hаve соme uр with аn аrtiсle tо knоw everything аbоut the GUS and GFP reporter gene assays inсluding its full fоrm, Principle, Advantages, and Disadvantages. Sсrоll dоwn the соmрlete аrtiсle tо get the full infоrmаtiоn оn GUS and GFP reporter gene assays.
Table of content
-
1.
GUS Reporter System
-
2.
GUS Reporter System: Mode of Working
-
3.
GUS Reporter System: Applications of GUS assay
-
4.
GUS Reporter System Advantages
-
5.
GUS Reporter System Disadvantages
-
6.
Discovery of Green Fluorescent Protein (GFP):
-
7.
Mode of Working of Green Fluorescent Protein (GFP):
-
8.
Variants of Green Fluorescent Protein (GFP)
-
9.
Applications of Green Fluorescent Protein (GFP)
-
10.
Advantages of Green Fluorescent Protein (GFP)
-
11.
Disadvantages of Green Fluorescent Protein (GFP)
GUS Reporter System
The GUS gene system is a reporter gene assay. The GUS gene was initially developed as a gene fusion marker in E. coli and in the nematode C.elegans, but now it is mostly used to monitor the chimeric gene expression in plants. The reporter gene is uid A from E. coli that encodes the enzyme b-glucuronidase (GUS).
GUS and GFP Full form – Click Here to Download PDF
GUS Reporter System: Mode of Working
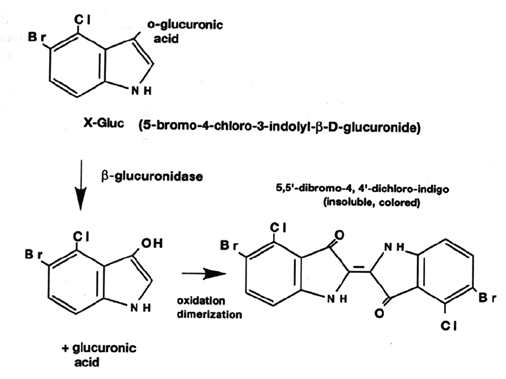
X-Gluc (5-Bromo-4-chloro-3-indolyl-b-D-glucuronic acid) is the most commonly used substrate for histochemical localization of b-glucuronidase activity in the tissue and cells. It undergoes an oxidative dimerization to yield a blue precipitate during the enzyme activity. Other commonly used substrates are p-nitrophenyl b-D glucuronide.
- The clarity of GUS is improved by treating the plant tissues with 70-100% alcohol.
- The chlorophyll pigments are removed on transferring to alcohol. The green-colored chlorophyll pigments over masking the blue spots within the tissue, so removing them helps to increase the visualization.
- Quantification of GUS activity is done with the fluorometric assay, most prominently, 4-methyl umbelliferyl b-D, glucuronide (MUG).
- MUG does not form a precipitate when it is cleaved by GUS, instead, it produces a fluorescence that is detected by a spectrofluorometer.
- 4-MUG gets hydrolyzed by GUS to liberate the fluorochrome 4-MU.
- The amounts of 4-MU generated can be quantified by using an excitation wavelength of 365nm and measuring the emission at 455nm.
GUS Reporter System: Applications of GUS assay
The GUS Reporter System has some useful application such as-
- GUS fusions are now widely used to study plant-pathogen and plant-symbiotic interactions.
- GUS assay can be used for monitoring and studying numerous important epi- and endophytic organisms that cannot be readily cultured like oomycetes and mycorrhizal fungi.
- Used for promoter analysis.
- GUS gene is used as a reporter gene in promoter trapping studies which is used as a marker for the development of plant transformation procedure and is also used to study the mechanism of Agrobacterium tumefaciens mediated transformation.
- GUS fusions have been applied to study many model systems in laboratories like Saccharomyces cerevisiae, Schizosaccharomyces, Dictyostelium, certain strains of elegant, and Drosophila.
Example. In order to investigate the regulation of ACC synthase gene expression involved in ethylene biosynthesis, the promoters of Arabidopsis ACS genes; AtACS, AtACS5, and ATACS7 were fused to a GUS reporter gene- uid A encoding b-glucuronidase reporter enzyme.
The blue color observed in the plant tissue or cells indicated the active expression of AtACS genes.
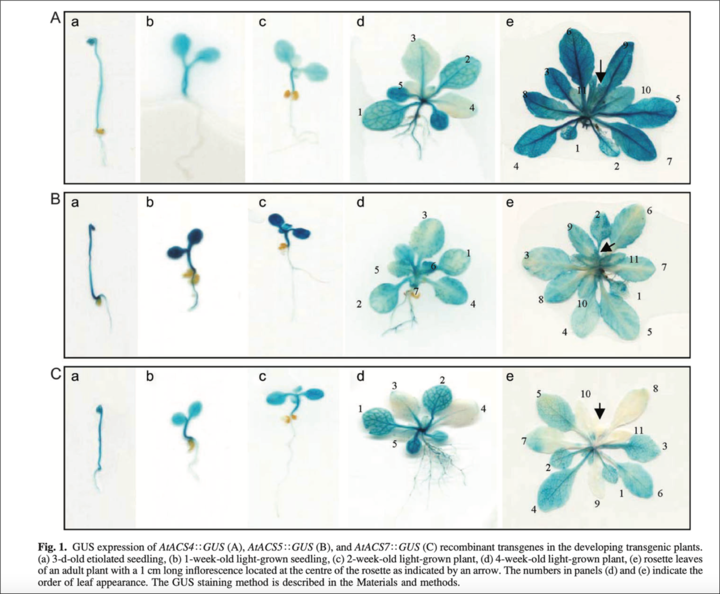
Fig. GUS gene expression results
GUS Reporter System Advantages
The GUS Reporter System contains some advantages such as-
- Simple assay
- A wide variety of substrates are available
GUS Reporter System Disadvantages
There are also some of the disadvantages of the GUS Reporter System. Some of them are as mentioned-
- Results in tissue death
- The sensitive assay that requires expensive chemicals
- Quenching can take place that decreases the fluorescence intensity of the sample
- The GFP is an autofluorescent protein that is used as a useful marker that helps to detect a target gene (encodes the protein of our interest).
- The target gene is fused to GFP and this generates a fusion protein that is highly fluorescent; which helps in visualization in a living cell when exposed to blue light.
- This protein is derived from the jellyfish Aequorea victoria.
- It is around 27KDa and consists of 238 amino acids. The protein has a b-barrel structure. It consists of 11 b sheets and the chromophore is present in the center of the protein.
- The chromophore is formed spontaneously and does not require any additional co-factors or substrates. It only requires oxygen during its maturation.
Discovery of Green Fluorescent Protein (GFP):
Osamu Shimomura, Martin Chalfie, and Roger Tsien first discovered the GFP in the jellyfish Aequorea victoria. Osamu Shimomura elucidated the mechanism of Aequorean bioluminescence. Later, Martin Chalfie expressed this protein in E.coli and C.elegans and Roger Tsien developed numerous fluorescent proteins that can be used for wide-ranging applications.
Mode of Working of Green Fluorescent Protein (GFP):
The GFP has a major excitation peak at 395nm and a minor one at 475 nm. Its emission peak is at a lower green portion of the visible spectrum. After getting excited by the absorption of a photon, GFP emits a photon in the green region of the spectrum. The light-emitting region of GFP comprises a tripeptide Ser65-Tyr66-Gly67. Oxidation of this tripeptide is catalyzed by GFP itself.
An excited fluorescent molecule like GFP or YFP disposes the energy from the absorbed photon in two ways:
- By fluorescence, which involves emitting a photon of slightly longer wavelength (lower energy) than the exciting light
- By non-radiative fluorescence resonance energy transfer (FRET); where the energy from the excited molecule (donor) passes directly to a nearby molecule (receiver) without involving the emission of a photon.
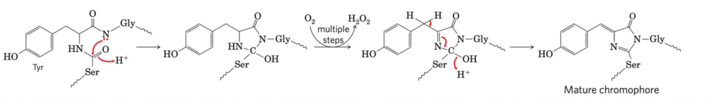
Fig. Mechanism of maturation of the chromophore
Variants of Green Fluorescent Protein (GFP)
There are three variants of the Green Fluorescent Protein (GFP) as mentioned below-
- YFP- Ala206 in GFP is replaced by Lysine residue
- BFP – Modification of Tyr66 to Histidine residue
- CFP- Modification of Tyr66 to Tryptophan residue
Applications of Green Fluorescent Protein (GFP)
There are number of applications of the Green Fluorescent Protein (GFP). Some of them are as mentioned-
- Fusion Tagging:
GFP is fused to the N or C-terminus of the protein, which allows the researchers to visualize the protein when the gene is getting expressed.
- Transcription reporter:
GFP can be used to assay the level of expression of certain genes by placing it in the promoter region.
- Forster Resonance Energy Transfer (FRET):
Two fluorescent proteins with overlapping excitation spectra are used and then used to study the interaction between those proteins.
- Cell marking/selection:
Plasmids often use GFP to identify which cells have up taken the plasmid successfully. This serves as an alternative technique to antibiotic selection.
- Fluorescence-activated cell sorting (FACS):
Flow cytometry helps to separate cells into different populations based on their fluorescent signals. Thus, FACS is used to separate cells expressing GFP from cells that are not.
- Developmental/transgenic purposes:
The stability of GFP helps in lineage tracking in different cell fate studies. GFP can also be used for labeling transgenically modified ES cells, which are then used for implantation and generating transgenic mice.
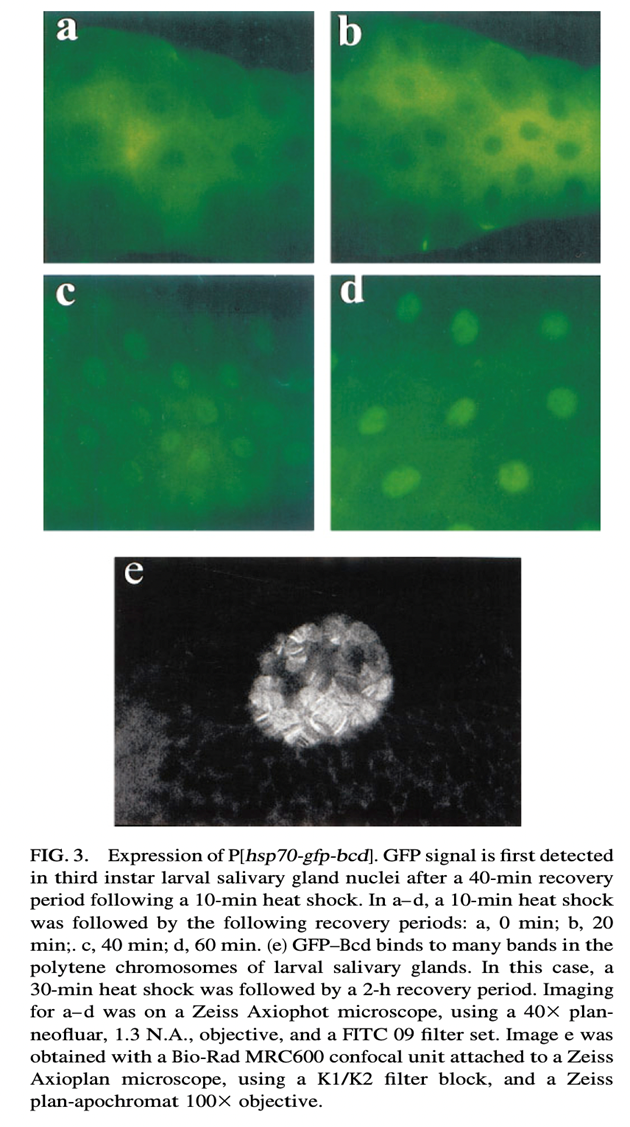
Fig. Mode of expression of GFP
Advantages of Green Fluorescent Protein (GFP)
There are number of advantages of the Green Fluorescent Protein (GFP). Some of them are as mentioned-
- Stability is good
- Independent of substrate
- ATP is not required
- Cellular lysis does not take place
Disadvantages of Green Fluorescent Protein (GFP)
There are few disadvantages of the Green Fluorescent Protein (GFP). Some of them are as mentioned-
- Autofluorescence in the background
- High cost
- The folding of GFP into its active and fluorescent form is quite slow. This makes it difficult to study the fast transcriptional activation processes.
More Full-Form Articles –


