- Home/
- CDS & Defence/
- Article
Give examples of Double displacement reactions.
By BYJU'S Exam Prep
Updated on: September 25th, 2023
A double displacement reaction is a particular type of reaction in which two reactants swap ions to produce two new molecules. A twofold replacement reaction is described by the general equation. The example of double displacement reaction is:
AgNO3 (s) + HCl (l) ⇔ AgCl (s) + HNO3 (l)
Table of content
Double Displacement Reaction
- Water-soluble ionic compounds interact with one another in a number of double displacement processes.
- Checking to see if the cations exchanged anions with one another is the simplest technique to determine whether a reaction is a double displacement. If the states of matter are mentioned, another indicator to observe for is the production of a single solid product and watery reactants (as the reaction mainly produces a precipitate).
- Aqueous metathesis with precipitation (precipitation reactions), counter-ion exchange, neutralisation, acid-carbonate reactions, alkylation, and aqueous metathesis with double decomposition are a few categories into which double displacement processes can be divided. Precipitation reactions and neutralisation reactions are the two types that are most frequently seen in chemistry lectures.
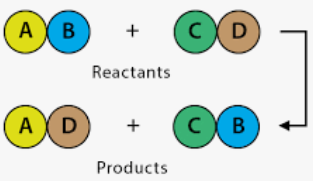
Example of Double Displacement Reaction
AgNO3 (s) + HCl (l) ⇔ AgCl (s) + HNO3 (l)
Summary:
Give examples of Double displacement reactions.
The double displacement reaction example is AgNO3 (s) + HCl (l) ⇔ AgCl (s) + HNO3 (l). A double displacement reaction is a special type of reaction in which two reactants exchange ions to create two new molecules.
