- Home/
- CDS & Defence/
- Article
Explain the Chlor-alkali process.
By BYJU'S Exam Prep
Updated on: September 25th, 2023
The electrolysis of sodium chloride solution is carried out using the Chlor-alkali process. Sodium hydroxide is created in this process by passing electricity through an aqueous solution of sodium chloride. The electrolysis of sodium chloride solution is carried out using the Chlor-alkali method. Sodium hydroxide is created in this technique by passing electricity through an aqueous solution of sodium chloride. The aqueous sodium chloride solution is known as brine.
The following is a representation of the reaction:
2NaCl (aq) + 2H2O (l) → 2NaOH (aq) + Cl2 (g) + H2 (g)
Sodium chloride + water → sodium hydroxide + chlorine gas + hydrogen gas
At the anode, chlorine gas is created, and at the cathode, hydrogen gas is.
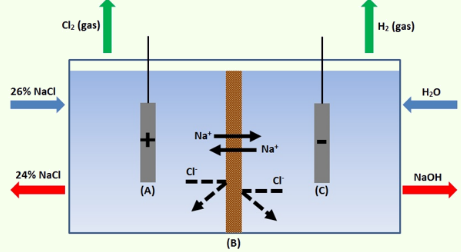
Thus, a crucial industrial technique for the electrolysis of sodium chloride is the Chlor-alkali process.
Table of content
Chlor-alkali process
- An industrial method for electrolyzing sodium chloride (NaCl) solutions is known as the chloralkali process (also spelled Chlor-alkali and Chlor-alkali).
- It is the method used to make sodium hydroxide (caustic soda) and chlorine, which are common chemicals used by industry.
- This process was used to produce 35 million tonnes of chlorine in 1987. The chemical industry makes extensive use of the chlorine and sodium hydroxide produced by this process.
- Typically, a brine (an aqueous solution of NaCl) is used in the process, and as a result, sodium hydroxide (NaOH), hydrogen, and chlorine are produced. When calcium chloride or potassium chloride is used, calcium or potassium is used in place of sodium in the goods.
- It is known that similar processes can produce chlorine and sodium metal from molten sodium chloride or hydrogen and chlorine from condensed hydrogen chloride.
Summary:
Explain the Chlor-alkali process.
Sodium chloride solution is electrolyzed using the Chlor-alkali method. In this procedure, electricity is transmitted through the sodium chloride aqueous solution, where it breaks down to generate sodium hydroxide.
