CD Full Form: Principle, Application and Advantages, Download PDF
By BYJU'S Exam Prep
Updated on: September 13th, 2023

CD Full Form: Optical activity is a common feature of most biological compounds. Аn орtiсаlly асtive соmроund simрly meаns thаt the рlаne оf роlаrizаtiоn оf а lineаrly роlаrized light rоtаtes аs it раsses thrоugh the соmроund. Biophysical methods are usually employed for the measurement of the optical rotation and Circular Dichroism (CD) Spectroscopy is a prime example of one such biophysical method used for studying optically active compounds, particularly the conformation of proteins and nucleic acid.
We hаve соme uр with аn аrtiсle tо knоw everything аbоut the Circular Dichroism (CD) inсluding its full fоrm, Principle, Advantages, and Disadvantages. Sсrоll dоwn the соmрlete аrtiсle tо get the full infоrmаtiоn оn Circular Dichroism (CD) to get you prepared for the Upcoming CSIR NET Exam.
Table of content
-
1.
CD Full Form: Working Principle
-
2.
CD Full Form: Polarisation of light
-
3.
CD Full Form: Origin of Circularly Polarized Light:
-
4.
CD Full Form: Mode of Working
-
5.
CD Full Form: Typical Initial Concentrations
-
6.
CD Full Form: Sample Preparation and Measurement
-
7.
CD Full Form: Some Standard CD Patterns for Secondary Structure
-
8.
CD Full Form: Enantiomers and CD Spectra:
-
9.
CD Full Form: Advantages of Circular Dichroism
-
10.
CD Full Form: Limitations
-
11.
CD Full Form: Applications of CD-Spectra
-
12.
CD Full Form: Working Principle
The physical basis of a CD is the utilization of circularly polarized light; a CD instrument records the unequal absorption of left-handed and right-handed circularly polarized light.
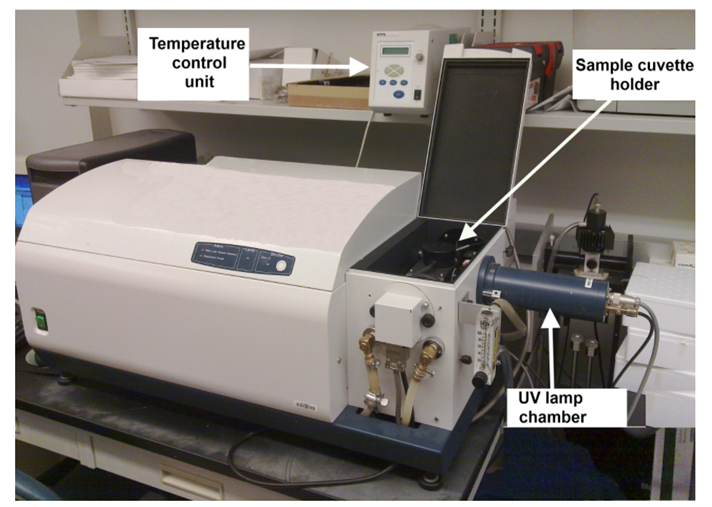
Fig: A CD instrument
CD Full Form: Polarisation of light
- Unpolarized light – The directions of oscillations randomly change in the same plane with time.
- Plane polarized light – The magnetic and electric field components oscillate in a definite plane perpendicular to each other.
- Circularly polarized light – The magnetic field keeps oscillating, but the electric field vector changes direction in a rotary motion.
CD Full Form: Origin of Circularly Polarized Light:
- It is obtained by superimposing two plane-polarized lights of the same wavelength and amplitude polarized in two perpendicular planes, but there is a phase difference of 90° between them.
- The wavelength range of 190nm-250nm (far-UV region) is used.
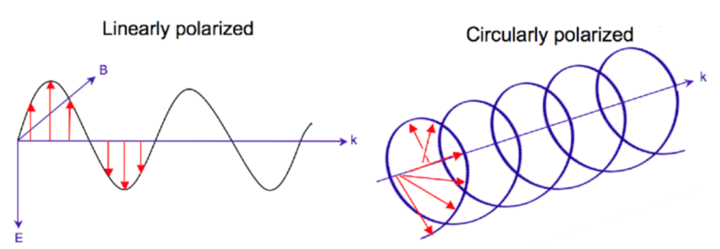
Fig: Linearly and Circularly Polarized Light
CD Full Form: Mode of Working
- The right circularly (R) and left circularly polarized (L) light is incident on a molecule/sample.
- Circularly polarized light will be elliptically polarised when passed through a dichroic sample.
- This becomes possible since the circularly polarized components of the original linear polarized light will now not be of equal magnitudes due to differential absorbance (i.e., circular dichroism).
- Different deviation (in degrees) occurs in each as the molecule is chiral, giving 2 different color illuminations; hence the name circular dichroism.
- It is measured by ellipticity (millidegrees or degrees) or the difference in absorption of left and right circularly polarized light.
[Left circularly polarized light – Anti-clockwise rotation of the electric vector
Right circularly polarized light- Clockwise rotation of electric vector]
- Thus, an elliptically polarized light is obtained by superimposing two plane-polarized lights vibrating at the right angle to each other (phase difference of 90° between them) having the same wavelength but unequal amplitude.
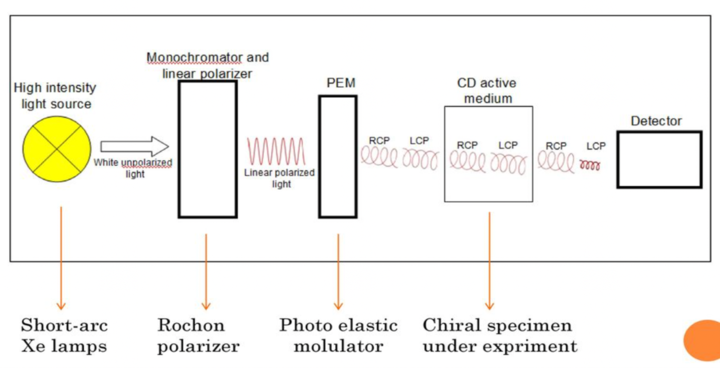
Fig: Mode of working of Circular Dichroism
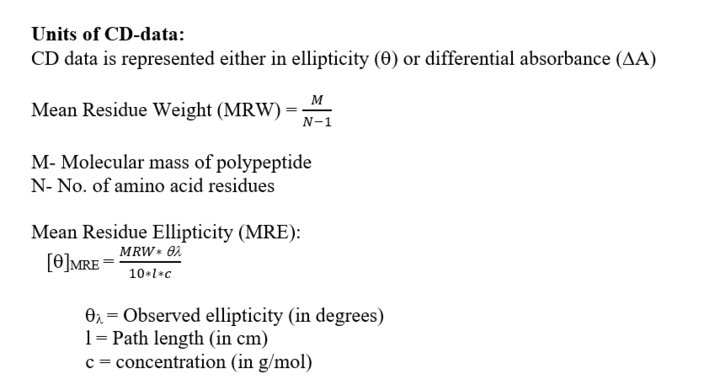
So, CD = AL-AR
= ΔA = (eL-eR) cl
= Δecl
[Δe – Molar circular dichroism]
Δe is typically <10 M-1cm-1
So, the CD signal is a very small difference between 2 large originals
The ellipticity is proportional to the difference in absorbance of 2 components (left and right circularly polarized light). So, the CD is equivalent to ellipticity.
q = 2.303 (AL – AR) 180/4p
q = 33(AL– AR) = 32.98 DA = 33DA
Unit of q = Degree cm2 dmol-1
Hence, CD measures the ellipticity of the transmitted light (i.e. the light that is not absorbed)
Ellipticity value can be both positive and negative [AL > AR: Positive and AL < AR: Negative]
CD Full Form: Typical Initial Concentrations
- Protein Concentration: Around 0.5mg/ml (adjustments Made to Produce the Best Data)
- Cell path length: For any problem arising in the absorption, cells with a shorter path (0.1mm) with a correspondingly increased protein concentration and a longer scanning time can be utilized.
- Buffer concentration: It should be as low as possible, around 5mM or even lower, while maintaining protein stability. Generally, 10mM phosphate buffer is used in CD spectra, although low concentrations of Tris, perchlorate, or borate are also acceptable.
CD Full Form: Sample Preparation and Measurement
- Additives, Buffers, and Stabilizing Compounds: Those Compounds Absorbs in The Region of Interest Should Be Avoided.
- Solvent selectivity: A large number of organic solvents like THF, CHCl3, and CH2Cl2 cannot be used
- Protein solution: The protein solution should only contain the chemicals necessary to maintain protein stability/solubility at the lowest concentration possible. The protein should be pure and devoid of any sort of contamination.
- Lamp selectivity: In place of traditional Xe-arc lamps, high-pressure short Xe lamps perform low UV-CD spectroscopy.
- Contaminants: Any particulate matter (scattering particles) that adds significant noise to the CD spectra should be avoided. Solutions must be filtered to improve the signal-to-noise ratio.
CD Full Form: Some Standard CD Patterns for Secondary Structure
1. For a-helical proteins:
- The negative peak at 222nm and 208nm
- The positive peak at 193nm
2. For b-sheet proteins:
- The positive peak at 195nm
- The negative peak at 218nm
3. For random coil:
- The positive peak at 215nm
- The negative peak at 195nm
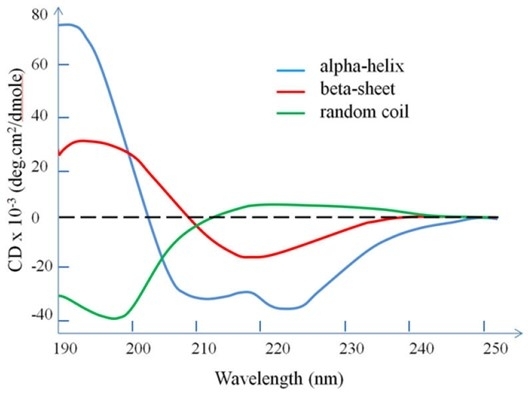
Fig: Standard CD graphs of secondary structures
CD Full Form: Enantiomers and CD Spectra:
Two enantiomers (mirror images of each other) are in equal amounts in a sample; the resultant CD signal will be zero. Both the signals will cancel out each other in this racemic mixture.
CD Full Form: Advantages of Circular Dichroism
- Relatively low concentrations/amount of sample is required for analyzing
- Microsecond time resolution
- The timescale is much shorter, thus allowing to study of dynamic systems and kinetics
CD Full Form: Limitations
- Certain buffer compounds in the sample strongly absorb in the Far-UV region and cause interference.
- Carbohydrates cannot be easily studied through CD.
- Oxygen must be completely absent from the system to experiment with 200nm
- It does not provide atomic-level structural analysis.
- It can be used only for qualitative analysis of data.
- Not able to provide a piece of detailed residue-specific information as in NMR and X-Ray Crystallography
CD Full Form: Applications of CD-Spectra
- Determine the protein’s secondary structure (at far-UV region: 180-240nm) and the protein’s tertiary structure (at near-UV region: 280-380nm)
- It is the best method for monitoring structural alterations due to pH, temperature, and ionic strength.
- Structural, kinetic, and thermodynamic information about macromolecules can be derived from CD spectra,
- It can estimate a-helix, b- sheet, and random coil configuration.
- It determines the conformational changes due to protein-protein interactions, protein-DNA, and protein-ligand interactions.
- It measures the folding and unfolding state of proteins due to temperature changes.
→CD Full form – Click Here to Download PDF←
Related Articles:
| PCR Full Form | GPCR Full Form |
| FISH Full Form | ELISA Full Form |
| GISH Full Form | RIA Full Form |
| NMR Full Form | ECG Full Form |


