- Home/
- CDS & Defence/
- Article
Capillary action is due to: _____? (A) Adhesion (B) Cohesion (C) Adhesion and cohesion both (D) Neither adhesion nor cohesion
By BYJU'S Exam Prep
Updated on: September 25th, 2023
Capillary action is due to adhesion and cohesion both. It has been discovered that when a tube with a very narrow bore—referred to as a capillary—is submerged in a liquid, the liquid inside either ascend or lowers in relation to the surrounding liquid. It is known as capillarity for this phenomenon.
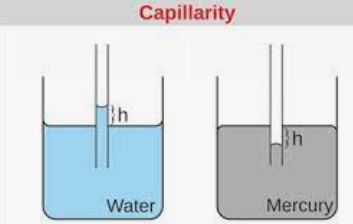
Table of content
Explain Capillarity
The differential in pressures between the concave and convex sides of the liquid’s curved surface is what causes capillarity. We have mentioned some points about capillarity to understand this important topic.
- The cohesiveness of a liquid’s molecules is what gives it’s surface the ability to withstand an external force.
- The phenomenon known as surface tension is caused by the cohesive forces between liquid molecules.
- Water molecules form a strong link with one another due to the forces of cohesion and adhesion, where they are drawn to and adhere to other materials like glass or paper.
Summary:
Capillary action is due to: _____? (A) Adhesion (B) Cohesion (C) Adhesion and cohesion both (D) Neither adhesion nor cohesion
Both adhesion and cohesion are responsible for capillary action. It has been found that the liquid inside a tube with a highly narrow bore, known as a capillary, either descends or ascends in respect to the surrounding liquid. This phenomenon is known as capillarity.
