Cannizzaro reaction is not given by? (A) Trimethylacetaldehyde (B) Acetaldehyde (C) Benzaldehyde (D) Formaldehyde
By BYJU'S Exam Prep
Updated on: October 17th, 2023
Cannizzaro reaction is not given by Acetaldehyde. Aldehydes that lack alpha hydrogen go via the Cannizaro reaction. Here, reduction and self oxidation occur in the presence of a concentrated aqueous potassium hydroxide solution or an alcoholic solution. Salt of carboxylic acid and alcohol are the products created.
Table of content
Stanislao Cannizzaro Gave Cannizzaro Reaction
Alpha hydrogen-containing compounds won’t produce the Cannizzaro reaction. Alpha hydrogen-deficient compounds will produce Cannizzaro’s reaction.
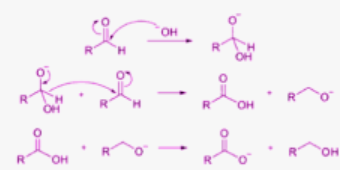
- The Cannizzaro reaction, named after Stanislao Cannizzaro, is a chemical reaction in which a base reacts disproportionately with two molecules of non-enolizable aldehyde to create a carboxylic acid and a primary alcohol.
- During a nucleophilic acyl substitution on an aldehyde, a leaving group attacks a separate aldehyde to cause the reaction. A tetrahedral intermediate is produced when hydroxide and carbonyl react. The carbonyl is reformatted, and a hydride is transferred, targeting a separate colony when this tetrahedral intermediate collapses
- According to the Cannizzaro Reaction Mechanism, two molecules of a certain aldehyde can be changed into one alcohol and one carboxylic acid molecule. Scientist Stanislao Cannizzaro succeeded in converting benzaldehyde into potassium benzoate and benzyl alcohol in 1853.
Summary:
Cannizzaro reaction is not given by? (A) Trimethyl acetaldehyde (B) Acetaldehyde (C) Benzaldehyde (D) Formaldehyde
Acetaldehyde does not cause the Cannizzaro reaction. Stanislao Cannizzaro gave Cannizzaro reaction. It was introduced in the year 1853. Succeeded in obtaining benzyl alcohol and potassium benzoate from benzaldehyde.


