- Home/
- SSC & Railways/
- SSC CGL/
- Article
Chemistry Notes on Acids and Base for Railways & SSC Exams 2023
By BYJU'S Exam Prep
Updated on: September 25th, 2023

Acid, Bases, and Salts: Acids, bases, and salts are important topics in chemistry that are fundamental to our understanding of chemical reactions and their applications in various fields. Acids are substances that release hydrogen ions (H+) when dissolved in water, while bases are substances that release hydroxide ions (OH-) when dissolved in water. Salts are ionic compounds formed by the reaction of an acid with a base, where the hydrogen ions of the acid are replaced by metal ions.
Some of the Acids, bases and salts are a part of our daily routine lives. For example, household cleaning products, cosmetics, and food preservation often involve the use of acids and bases. Understanding their properties, reactions, and safety precautions is important to handle and use them properly, ensuring the safety of oneself and others.
Table of content
Acid, Bases, and Salts
Chemistry is an integral part of Railway and SSC exams. Around 3 to 4 questions are based on Chemistry and one of the important topic is Acid, Bases, and Salts. But what does it mean? Read through the post to learn more about Acid, Bases, and Salts and the theories related to it.
Definition of Acid, Base and Salts
Acids: Acid is derived from the Latin word acidus, which means sour. Everything with a sour taste contains acid. Acids are sour-tasting chemicals. The acid solution has a pH of less than seven. Sour flavors can be found in lemon juice, tomatoes, and vinegar, for example. As a result, each of these substances must contain acid. Organic acids are acids found in both plants and mammals. Mineral acids are organic acids formed from Earth’s minerals. Acids contain a lot of hydrogen ions. As a result, an acid dissolves in water, producing a solution of hydrogen ions (H+).
Base: Bases are chemical compounds that have a harsh taste. Acids’ chemical opposites are bases. Any material that may neutralize acid is referred to as a base. All of the bases turn red and litmus blue. The pH of the basic solution is more than 7. Sodium hydroxide, magnesium hydroxide, calcium hydroxide, magnesium hydroxide, and other bases are examples. Alkalis include sodium hydroxide, potassium hydroxide, and calcium hydroxide. Hydrogen ions are found in bases. As a result, a base is a material that dissolves in water and produces hydroxide ions (OH-).
Salt: Salt is an acid-formed chemical in which a metal substitutes the acid hydrogen. Salts are formed when acids react with foundations. The pH of a salt solution is generally around 7. The most well-known salt is sodium chloride (NaCl), sometimes known as common salt. The act of deciding whether or not a person is responsible for his or her own acts is referred to as
esponsibility.
Theories Related to Acid, Base, and Salts
There are various theories that are associated with Acid and bases which are given by different scientists at different times. The most important theories related to Acids, Bases, and Salts are provided below:
Arrhenius’s concept of acids and bases
- Acid is a substance that produces hydrogen ions (H+) in an aqueous solution e.g. HCL (H+ Cl–), Sulphuric Acid, and H2SO4 (2H+ SO2-4).
- The base is a substance that produces Hydroxide ions (OH–) in an aqueous solution e.g. sodium hydroxide and ammonium hydroxide etc.
Bronsted Lowery concept of Acids and Bases
- An acid is a molecule or ion which is capable of donating a proton.
- A base is a molecule or ion which is capable of accepting a proton.
Lewis concept of Acids and Bases
- An acid is a substance which can accept an electron e.g. boron fluoride (BF3) and carbon dioxide.
- The base is a substance which can produce an electron e.g. fluoride (F–) and chloride (Cl–).
Acid, Bases, and Salts Examples
There are various examples of Acids, Bases, and Salts which may or may not be in daily life. Some important examples of Acids, Bases, and Salts are as follows:
Acids: Acetic Acid, Formic Acid, Citric Acid, Sulphuric Acid, Nitric Acid, etc.
Bases: Sodium Hydroxide, Potassium Hydroxide, Calcium Hydroxide, Ammonia Solution, etc.
Salts: Sodium Chloride (NaCl), Potassium Chloride, Sodium Sulphate, Potassium Sulphate, etc.
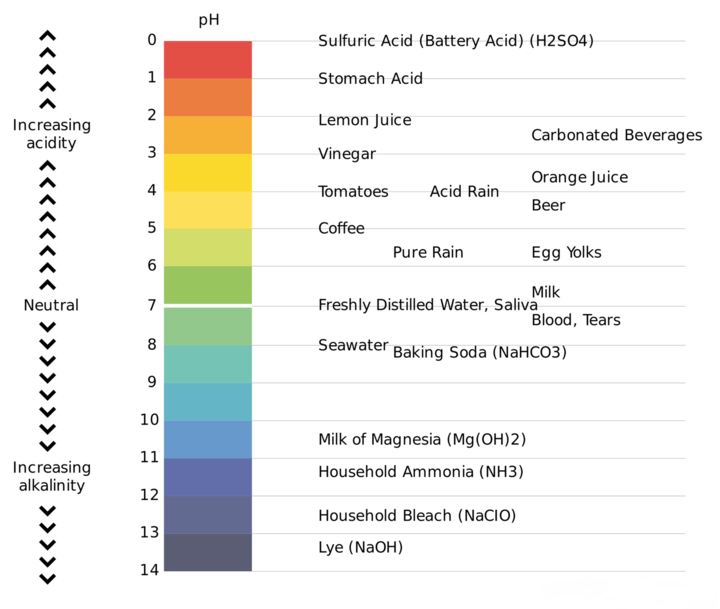
Important pH Values for Competitive Exams
What is the pH Scale?
- PH value is a measure of the acidity or basicity of an aqueous solution.
- A solution with a pH value less than 7 is considered acidic.
- A solution with a pH value greater than 7 is greater 7 is considered basic.
The below table contains some important Acids along with the chemical formula and the source of that particular acid.
|
Acid |
Chemical Formula |
Present in |
|
Acetic Acid |
CH3COOH |
Vinegar |
|
Formic Acid |
HCOOH |
Red ants |
|
Citric Acid |
C6H8O7 |
Citrus Fruits |
|
Lactic Acid |
C3H6O3 |
Curd |
|
Ascorbic Acid |
C6H8O6 |
Amla |
|
Tartaric Acid |
C₄H₆O₆ |
Grapes, Ripe Mangoes |
| Oxalic Acid | C2H2O4 |
Spinach |
pH values of some important solutions.
|
Substance |
pH Value |
|
Blood |
7.3 to 7.5 |
|
Tears |
7.4 |
|
Saliva |
6.5-7-5 |
|
Urine |
5.5-7.5 |
|
Coffee |
4.5-5.5 |
|
Beer |
4.0-5.0 |
|
Wine |
2.8-3.8 |
|
Vinegar |
2.4 -3.4 |
Acids in Fruits
Fruits and vegetables include acids such as acetic acid, citric acid, benzoic acid, malic acid, oxalic acid, and tartaric acid. Both fruits and vegetables contain necessary acids, but vegetables are naturally less acidic, making them a somewhat healthier source of essential acids if you have acid reflux or a high stomach acidity level. The only acids that can be consumed are those found in various foods. These acids are responsible for food’s strong flavor. Acids are categorized as having a pH level of less than 7 on a scale of 0 to 14. Acids can be found in a variety of plants, fruits, and dairy products. Some of these acids are nutrients, while others are treatments for specific ailments.
- Citrus fruits that contain citric acid include lemons, limes, and oranges. Pineapples, berries, grapes, tomatoes, pomegranates, and grape juice all contain it.
- Malic acid is abundant in apples. Broccoli, tomatoes, pomegranates, grapes, grapefruit, bananas, and grapes all contain it.
- Ascorbic acid, often known as Vitamin C, can be found in a variety of foods such as citrus fruits, berries, green leafy vegetables, green peppers, and tomatoes
- Tartaric acid can be found in apples, apricots, bananas, tamarind, grapes, and tamarind.
- Tartaric acid, malic acid, and citric acid are examples of natural dietary acids. They are also referred to as healthy acids. They can be found in seeds-containing fruits such as oranges, oranges, pineapples, apples, and peaches. Potatoes, grapes, and pineapples all contain tartaric acid. These fruits may taste bland if these acids are missing.
Buffer Solutions
Buffer solutions are those that withstand changes in pH caused by the addition of a little amount of acid or base. The pH of the acidic buffer solution is less than 7. A basic buffer has a pH higher than 7. Despite the fact that we eat a lot of acidic meals, the PH of our blood is kept stable with the help of an H2CO3/HCO3 buffer.

