- Home/
- CDS & Defence/
- Article
3p Orbital has _____ Radial Nodes
By BYJU'S Exam Prep
Updated on: September 25th, 2023
- three
- two
- one
- None
3p orbital has one radial nodes. This dual nature is likewise true of the electrons in an atom. Due to their small size and insignificant bulk, they have a substantial wave character. The electron behaves like a wave, as described by its orbital.
Table of content
Why 3p Orbital has one Radial Nodes
- The likelihood of finding an electron, symbolized by the symbol ‘ψ’, is calculated using the orbitals, which are mathematical functions.
- In these orbitals, the possibility of discovering an electron is greatest.
- The probability density of the electrons is given to us by the square of the wave functions ψ2.
- Two components comprise the wave functions:
- The radial component provides the orbitals’ spread or size.
- Giving us information about the orbitals’ orientation in space and angles.
- The electron density drops to zero at some points when the probability distribution function ψ2 is plotted versus the electrons’ distance from the nucleus
. Nodes are these locations where there is essentially little chance of detecting electrons. The plane is known as a nodal plane.
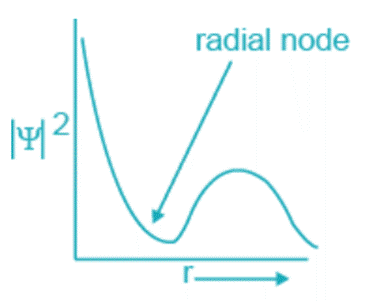
The formula n – l -1, where n is the primary quantum number and l is the azimuthal quantum number, yields the number of radial nodes.
The primary quantum number (n) for a 3p orbital is 3 and the azimuthal quantum number (l) for p is 1.
Thus, n – l -1 is equal to:
3 – 1 – 1 Equals 1.
As a result, the 3p orbital has one radial node.
Summary:-
3p Orbital has _____ Radial Nodes 1. three 2. two 3. one 4. None
One radial node is present in the 3p orbital. This dual nature of the electrons is true in an atom. Because of the insignificant bulk and small size, they contain a substantial wave character.
Related Questions:-
- Choose the Odd one out of the given Options. 1. PQH 2. HJL 3. GJM 4. ELM
- Moss and Ferns Reproduce by
- Sand Drains are Used to
- The Half-life Period of a Radioactive Substance Depends Upon
- Which One of the following is not a Search Engine?
- Who Among the following is the Person in Charge of the Police Station?
